A 8.17 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72 "C. After the salt has completely dissolved, the temperature of the solution is 28.54 "C. Was the dissolution process endothermic or exothermic? How much heat was gained by the solution? Assume the specific heat of the soluti d. is the same as water, 4.184 J/g "C. e What is the total heat for the dissolution reaction of the 8.17g of salt? How many moles of the unknown salt were used in the reaction? What is the enthalpy change (in kJ/mol of salt) for the dissolution reaction?
A 8.17 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72 "C. After the salt has completely dissolved, the temperature of the solution is 28.54 "C. Was the dissolution process endothermic or exothermic? How much heat was gained by the solution? Assume the specific heat of the soluti d. is the same as water, 4.184 J/g "C. e What is the total heat for the dissolution reaction of the 8.17g of salt? How many moles of the unknown salt were used in the reaction? What is the enthalpy change (in kJ/mol of salt) for the dissolution reaction?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 68E: In a coffee-cup calorimeter, 1.60 g NH4NO3 is mixed with 75.0 g water at an initial temperature of...
Related questions
Question
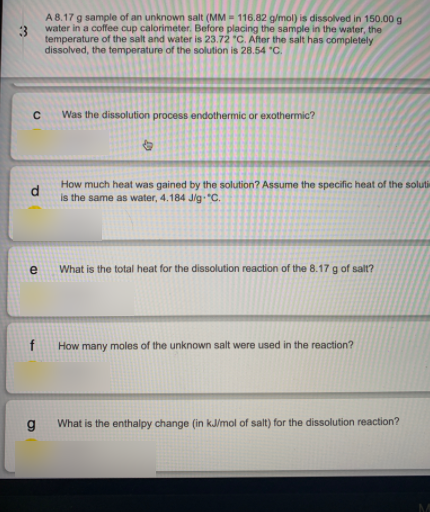
Transcribed Image Text:A 8.17 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g
water in a coffee cup calorimeter. Before placing the sample in the water, the
temperature of the salt and water is 23.72 "C. After the salt has completely
dissolved, the temperature of the solution is 28.54 "C.
Was the dissolution process endothermic or exothermic?
How much heat was gained by the solution? Assume the specific heat of the soluti
d.
is the same as water, 4.184 J/g "C.
e
What is the total heat for the dissolution reaction of the 8.17g of salt?
How many moles of the unknown salt were used in the reaction?
What is the enthalpy change (in kJ/mol of salt) for the dissolution reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning