Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH,CO_(29)+H,O) – H,O* (aq)-CH,CO,(1) Imagine 214. mmol of HCH,CO₂ are removed from a flask containing a mixture of HCH,CO₂, H₂O, H,O and CH,CO, at equilibrium, and then answer the following questions. What is the rate of the forward reaction before any HCH,CO, has been removed from the flask? What is the rate of the forward reaction just after the HCH,CO, has been removed from the flask? What is the rate of the forward reaction when the system has again reached equilibrium? How much less HCH,CO, is in the flask when the system has again reached equilibrium? Zero, Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction Zero Greater than zero, but less than the rate of the reverse reaction Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. None. Some, but less than 214, mmol., 214, mmol 114 mmol
Acetic acid and water react to form hydronium cation and acetate anion, like this: HCH,CO_(29)+H,O) – H,O* (aq)-CH,CO,(1) Imagine 214. mmol of HCH,CO₂ are removed from a flask containing a mixture of HCH,CO₂, H₂O, H,O and CH,CO, at equilibrium, and then answer the following questions. What is the rate of the forward reaction before any HCH,CO, has been removed from the flask? What is the rate of the forward reaction just after the HCH,CO, has been removed from the flask? What is the rate of the forward reaction when the system has again reached equilibrium? How much less HCH,CO, is in the flask when the system has again reached equilibrium? Zero, Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction Zero. Greater than zero, but less than the rate of the reverse reaction. Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction Zero Greater than zero, but less than the rate of the reverse reaction Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. None. Some, but less than 214, mmol., 214, mmol 114 mmol
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter23: Determination Of The Equilibrium Constant For A Chemical Reaction
Section: Chapter Questions
Problem 1ASA
Related questions
Question
F125.
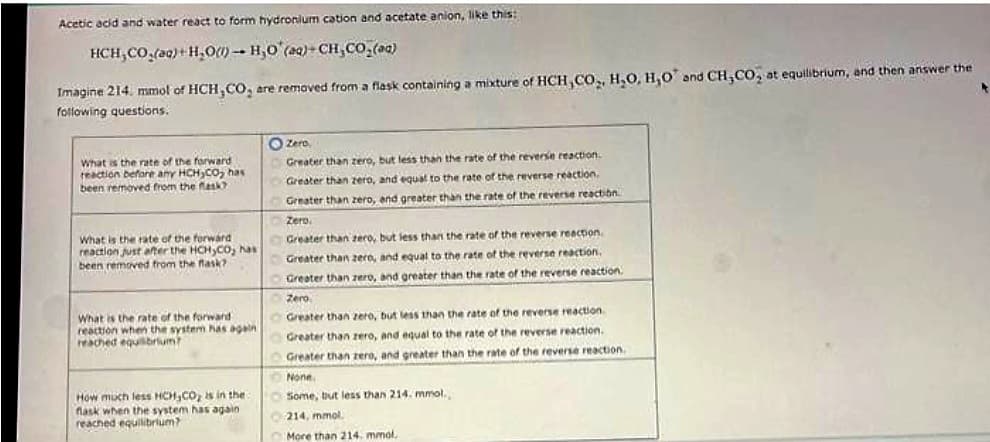
Transcribed Image Text:Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH,CO,ag}+H,O) – H,O (Q)-CH,CO_(1)
Imagine 214. mmol of HCH,CO₂ are removed from a flask containing a mixture of HCH,CO₂, H₂O, H,O and CH,CO, at equilibrium, and then answer the
following questions.
What is the rate of the forward
reaction before any HCH CO₂ has
been removed from the flask?
What is the rate of the forward
reaction just after the HCH₂CO; has
been removed from the flask?
What is the rate of the forward
reaction when the system has again
reached equilibrium?
How much less HCH,CO₂ is in the
flask when the system has again
reached equilibrium?
Zero,
Greater than zero, but less than the rate of the reverse reaction.
Greater than zero, and equal to the rate of the reverse reaction.
Greater than zero, and greater than the rate of the reverse reaction
Zero.
Greater than zero, but less than the rate of the reverse reaction.
Greater than zero, and equal to the rate of the reverse reaction.
Greater than zero, and greater than the rate of the reverse reaction.
Zero
Greater than zero, but less than the rate of the reverse reaction
Greater than zero, and equal to the rate of the reverse reaction.
Greater than zero, and greater than the rate of the reverse reaction.
None.
Some, but less than 214. mmol.,
214, mmol
More than 214. mmol,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning