Acetone is a well-known industrial solvent with a molecular formula of: C3H60. Calculate for its formula weight? (Use the atomic masses from the description.) C-12.001 g/mol; H - 1.008 g/mol; 0 -15.999 g/mol 58.08 g/mol 80.58 g/mol 85.08 g/mol 80.85 g/mol Using the molecular weight from the previous question, what is the percentage by mass of carbon in acetone? 64.02% 20.68% 20.86% 62.04% Using the molecular weight from the previous question, what is the percentage by mass of hydrogen in acetone? 10.41% 17.31% 10.14% 17.13%
Acetone is a well-known industrial solvent with a molecular formula of: C3H60. Calculate for its formula weight? (Use the atomic masses from the description.) C-12.001 g/mol; H - 1.008 g/mol; 0 -15.999 g/mol 58.08 g/mol 80.58 g/mol 85.08 g/mol 80.85 g/mol Using the molecular weight from the previous question, what is the percentage by mass of carbon in acetone? 64.02% 20.68% 20.86% 62.04% Using the molecular weight from the previous question, what is the percentage by mass of hydrogen in acetone? 10.41% 17.31% 10.14% 17.13%
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 101A: Air Pollution Nitrogen monoxide, which is present in urban air pollution, immediately Converts to...
Related questions
Question
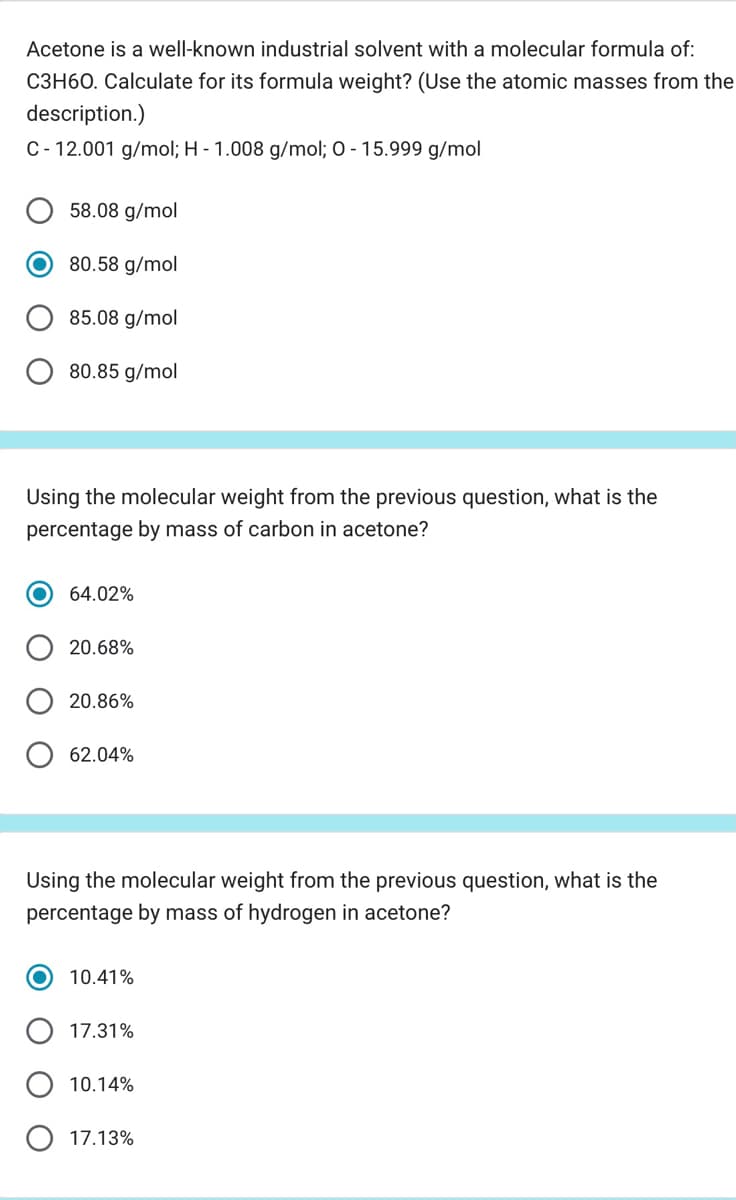
Transcribed Image Text:Acetone is a well-known industrial solvent with a molecular formula of:
C3H60. Calculate for its formula weight? (Use the atomic masses from the
description.)
C-12.001 g/mol; H - 1.008 g/mol; 0 -15.999 g/mol
58.08 g/mol
80.58 g/mol
85.08 g/mol
80.85 g/mol
Using the molecular weight from the previous question, what is the
percentage by mass of carbon in acetone?
64.02%
20.68%
20.86%
62.04%
Using the molecular weight from the previous question, what is the
percentage by mass of hydrogen in acetone?
10.41%
17.31%
10.14%
17.13%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning