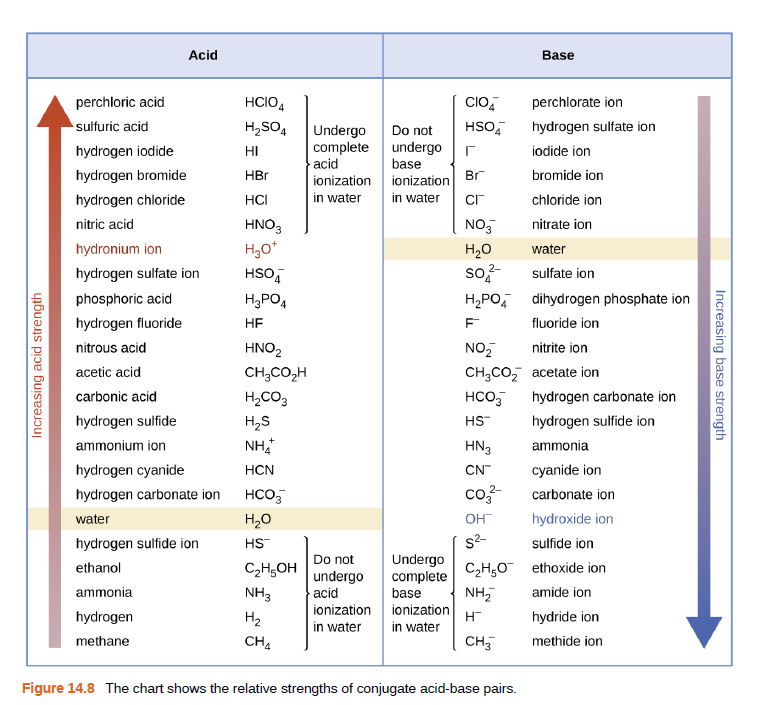
Acid Base perchloric acid HCIO, CIO, perchlorate ion sulfuric acid H2SO, HSO, hydrogen sulfate ion Undergo complete acid Do not hydrogen iodide undergo base HI iodide ion hydrogen bromide HBr ionization ionization Br bromide ion hydrogen chloride HCI in water in water Cr chloride ion nitric acid HNO3 NO, nitrate ion hydronium ion H20 water hydrogen sulfate ion HSO, so?- sulfate ion phosphoric acid H,PO4 H,PO, dihydrogen phosphate ion hydrogen fluoride HF fluoride ion nitrous acid HNO2 NO, nitrite ion CH;CO,H H,CO3 acetic acid CH,CO, acetate ion carbonic acid HCO5 hydrogen carbonate ion hydrogen sulfide H,S HS hydrogen sulfide ion ammonium ion NH HN3 ammonia hydrogen cyanide HCN CN cyanide ion hydrogen carbonate ion HCO, co- carbonate ion water H20 OH hydroxide ion hydrogen sulfide ion HS s2- sulfide ion Undergo complete base ionization ionization Do not ethanol C2H,OH C2H,O ethoxide ion undergo ammonia NH3 acid NH, amide ion hydrogen H2 in water H in water hydride ion methane CH4 CH, methide ion Figure 14.8 The chart shows the relative strengths of conjugate acid-base pairs. Increasing acid strength Increasing base strength
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
Using the Ka value of 1.4 × 10−5, place Al(H2 O)6 3+ in the correct location as shown.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps







