Amounts of iodine dissolved in aqueous solution, Iz(aq), can be determined by titration with thiosulfate ion (S2O³¯). The thiosulfate ion is oxidized to S40; while the iodine is reduced to iodide ion. Starch is used as an indicator because it has a strong blue color in the pres- ence of dissolved iodine. (a) Write a balanced equation for this reaction. (b) If 56.40 mL of 0.100 M S20;¯ solution is used to reach the endpoint of a titration of an unknown amount of iodine, calculate the number of moles of iodine originally present. (c) Combine the appropriate half-cell potentials from Appendix E with thermodynamic data from Appen- dix D for the equilibrium I2 (s)=1½(aq) to calculate the equilibrium constant at 25°C for the reaction in part (a).
Amounts of iodine dissolved in aqueous solution, Iz(aq), can be determined by titration with thiosulfate ion (S2O³¯). The thiosulfate ion is oxidized to S40; while the iodine is reduced to iodide ion. Starch is used as an indicator because it has a strong blue color in the pres- ence of dissolved iodine. (a) Write a balanced equation for this reaction. (b) If 56.40 mL of 0.100 M S20;¯ solution is used to reach the endpoint of a titration of an unknown amount of iodine, calculate the number of moles of iodine originally present. (c) Combine the appropriate half-cell potentials from Appendix E with thermodynamic data from Appen- dix D for the equilibrium I2 (s)=1½(aq) to calculate the equilibrium constant at 25°C for the reaction in part (a).
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 32PS: (a) Write equations for the half-reactions that occur at the cathode and the anode when an aqueous...
Related questions
Question

Transcribed Image Text:Amounts of iodine dissolved in aqueous solution, Iz(aq),
can be determined by titration with thiosulfate ion
(S2O³¯). The thiosulfate ion is oxidized to S40; while
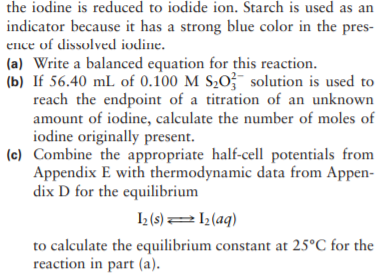
Transcribed Image Text:the iodine is reduced to iodide ion. Starch is used as an
indicator because it has a strong blue color in the pres-
ence of dissolved iodine.
(a) Write a balanced equation for this reaction.
(b) If 56.40 mL of 0.100 M S20;¯ solution is used to
reach the endpoint of a titration of an unknown
amount of iodine, calculate the number of moles of
iodine originally present.
(c) Combine the appropriate half-cell potentials from
Appendix E with thermodynamic data from Appen-
dix D for the equilibrium
I2 (s)=1½(aq)
to calculate the equilibrium constant at 25°C for the
reaction in part (a).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning