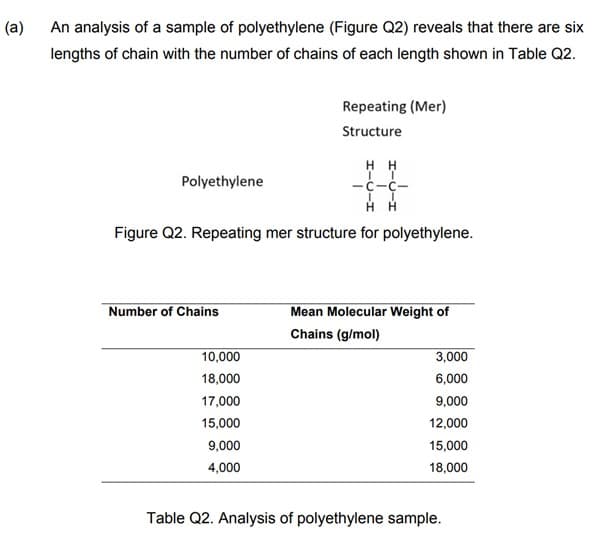
An analysis of a sample of polyethylene (Figure Q2) reveals that there are six lengths of chain with the number of chains of each length shown in Table Q2. HH TT -C-C- II Н Н Figure Q2. Repeating mer structure for polyethylene. Polyethylene Number of Chains Repeating (Mer) Structure 10,000 18,000 17,000 15,000 9,000 4,000 Mean Molecular Weight of Chains (g/mol) 3,000 6,000 9,000 12,000 15,000 18,000 Table Q2. Analysis of polyethylene sample.
An analysis of a sample of polyethylene (Figure Q2) reveals that there are six lengths of chain with the number of chains of each length shown in Table Q2. HH TT -C-C- II Н Н Figure Q2. Repeating mer structure for polyethylene. Polyethylene Number of Chains Repeating (Mer) Structure 10,000 18,000 17,000 15,000 9,000 4,000 Mean Molecular Weight of Chains (g/mol) 3,000 6,000 9,000 12,000 15,000 18,000 Table Q2. Analysis of polyethylene sample.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 110QRT
Related questions
Question
Determine:
(i) The weight average molecular weight and the degree of
polymerization.
(ii)The number average molecular weight and the degree of
polymerization.
(iii) What is the polydispersity index for this material?
(b) A sample of a polyethylene rope weighs 15.12 g per cm. If each chain
contains 7000 repeat units, calculate, the number of polyethylene chains in
a 3 m rope.
Transcribed Image Text:(a)
An analysis of a sample of polyethylene (Figure Q2) reveals that there are six
lengths of chain with the number of chains of each length shown in Table Q2.
Polyethylene
Number of Chains
Repeating (Mer)
Structure
II
HH
Figure Q2. Repeating mer structure for polyethylene.
10,000
18,000
17,000
15,000
9,000
4,000
HH
IT
-c-c-
Mean Molecular Weight of
Chains (g/mol)
3,000
6,000
9,000
12,000
15,000
18,000
Table Q2. Analysis of polyethylene sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Introduction
VIEWStep 2: Calculation of the weight average molecular weight
VIEWStep 3: Calculation of the degree of polymerization
VIEWStep 4: (b) Calculation of number average molecular weight
VIEWStep 5: Calculation of the degree of polymerization
VIEWStep 6: Calculation of polydispersity index
VIEWSolution
VIEWStep by step
Solved in 7 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning