An automobile engine provides 565 Joules of work to push the pistons. In this process the internal energy changes by -2694 Joules. Calculate q for the engine. This represents the amount of heat that must be carried away by the cooling system. q = Joules
An automobile engine provides 565 Joules of work to push the pistons. In this process the internal energy changes by -2694 Joules. Calculate q for the engine. This represents the amount of heat that must be carried away by the cooling system. q = Joules
Chapter10: Energy
Section: Chapter Questions
Problem 28A
Related questions
Question
1
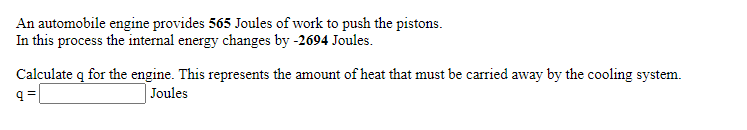
Transcribed Image Text:An automobile engine provides 565 Joules of work to push the pistons.
In this process the internal energy changes by -2694 Joules.
Calculate q for the engine. This represents the amount of heat that must be carried away by the cooling system.
Joules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning