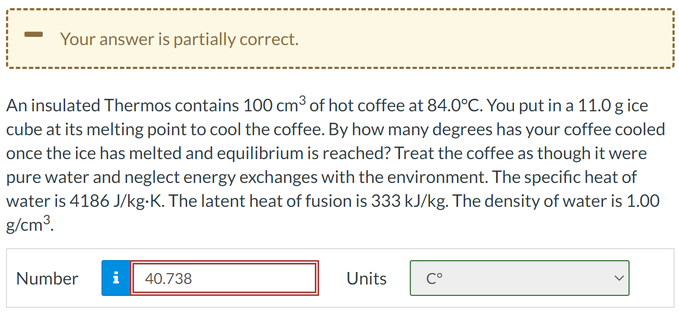
An insulated Thermos contains 100 cm³ of hot coffee at 84.0°C. You put in a 11.0 g ice cube at its melting point to cool the coffee. By how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00 g/cm³. Number i 40.738 Units Cº
An insulated Thermos contains 100 cm³ of hot coffee at 84.0°C. You put in a 11.0 g ice cube at its melting point to cool the coffee. By how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00 g/cm³. Number i 40.738 Units Cº
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 21PQ
Related questions
Question
answer needs 3 significant figures. please help with solution.
thanks
thanks
Transcribed Image Text:Your answer is partially correct.
An insulated Thermos contains 100 cm³ of hot coffee at 84.0°C. You put in a 11.0 g ice
cube at its melting point to cool the coffee. By how many degrees has your coffee cooled
once the ice has melted and equilibrium is reached? Treat the coffee as though it were
pure water and neglect energy exchanges with the environment. The specific heat of
water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00
g/cm³.
Number
Mi
40.738
Units
Cº
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning