Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.86PAE: Consider two samples of liquid: 1 mole of water (H2O) and 1 mole of ethanol (C2H50H). Answer each of...
Related questions
Question
100%
Transcribed Image Text:Student Information
Chem159 - Practice 5 Compounds
No. of Questions
Work Tir
Liam A Fox (laf251)
Practice
20
297:49:4
1
2
4.
6.
7
8
9.
10
11 12
13
14
15
16
18
19
20
il.
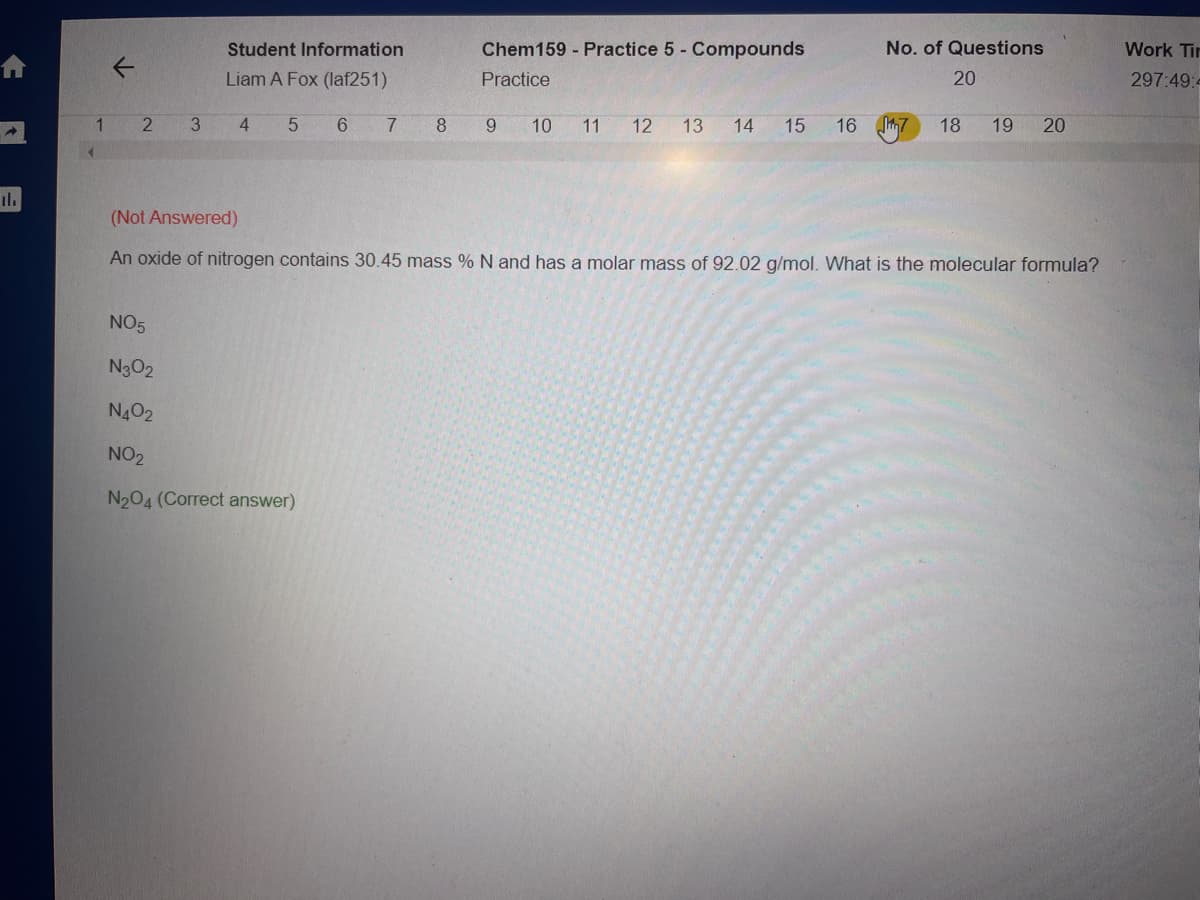
(Not Answered)
An oxide of nitrogen contains 30.45 mass % N and has a molar mass of 92.02 g/mol. What is the molecular formula?
NO5
N302
N402
NO2
N204 (Correct answer)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning