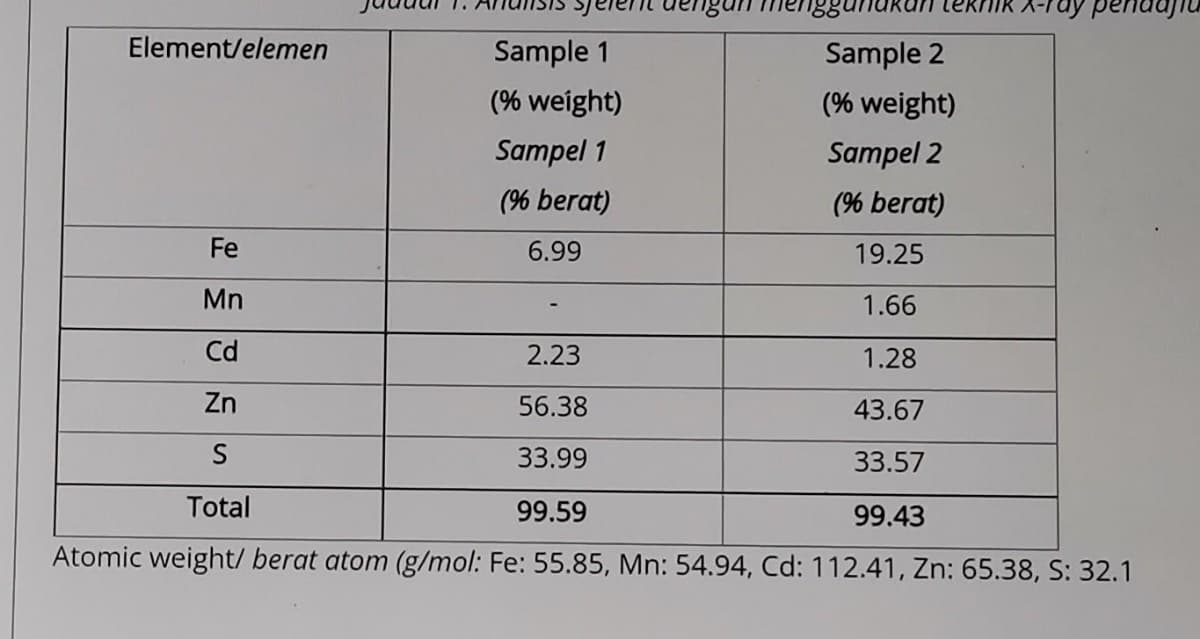
Analysis of sphalerite using X-ray Fluorescence technique is shown in Table 1. Sphalerite may be nearly pure zinc sulfide or, it may contain considerable quantities of iron and minor amounts of manganese and cadmium. This phenomenon is common in minerals, and it can be indicated by writing the formula as (Zn, Fe)S, which shows that total of Zn+Fe is 1 with respect to S=1, but actual amounts of Zn and Fe are variable. How would you explain this situation by mineral formula calculation
Analysis of sphalerite using X-ray Fluorescence technique is shown in Table 1. Sphalerite may be nearly pure zinc sulfide or, it may contain considerable quantities of iron and minor amounts of manganese and cadmium. This phenomenon is common in minerals, and it can be indicated by writing the formula as (Zn, Fe)S, which shows that total of Zn+Fe is 1 with respect to S=1, but actual amounts of Zn and Fe are variable. How would you explain this situation by mineral formula calculation
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 25QAP: Cyclopropane mixed in the proper ratio with oxygen can be used as an anesthetic. At 755 mm Hg and...
Related questions
Question
Analysis of sphalerite using X-ray Fluorescence technique is shown in Table 1. Sphalerite may be nearly pure zinc sulfide or, it may contain considerable quantities of iron and minor amounts of manganese and cadmium. This phenomenon is common in minerals, and it can be indicated by writing the formula as (Zn, Fe)S, which shows that total of Zn+Fe is 1 with respect to S=1, but actual amounts of Zn and Fe are variable. How would you explain this situation by mineral formula calculation?
Transcribed Image Text:dengan me
ay pend
Element/elemen
Sample 1
Sample 2
(% weight)
(% weight)
Sampel 1
Sampel 2
(% berat)
(% berat)
Fe
6.99
19.25
Mn
1.66
Cd
2.23
1.28
Zn
56.38
43.67
S
33.99
33.57
Total
99.59
99.43
Atomic weight/ berat atom (g/mol: Fe: 55.85, Mn: 54.94, Cd: 112.41, Zn: 65.38, S: 32.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning