Aqueous A reacts to form R ( A R ) and in the first minute in a batch reactor its concentration drops from CA0 = 2.03 mol/liter to C = 1.97 mol/liter. Find the rate equation for the reaction if the kinetics are second order with respect to A.
Aqueous A reacts to form R ( A R ) and in the first minute in a batch reactor its concentration drops from CA0 = 2.03 mol/liter to C = 1.97 mol/liter. Find the rate equation for the reaction if the kinetics are second order with respect to A.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Aqueous A reacts to form R ( A R ) and in the first minute in a batch reactor its concentration drops from CA0 = 2.03 mol/liter to C = 1.97 mol/liter. Find the rate equation for the reaction if the kinetics are second order with respect to A.
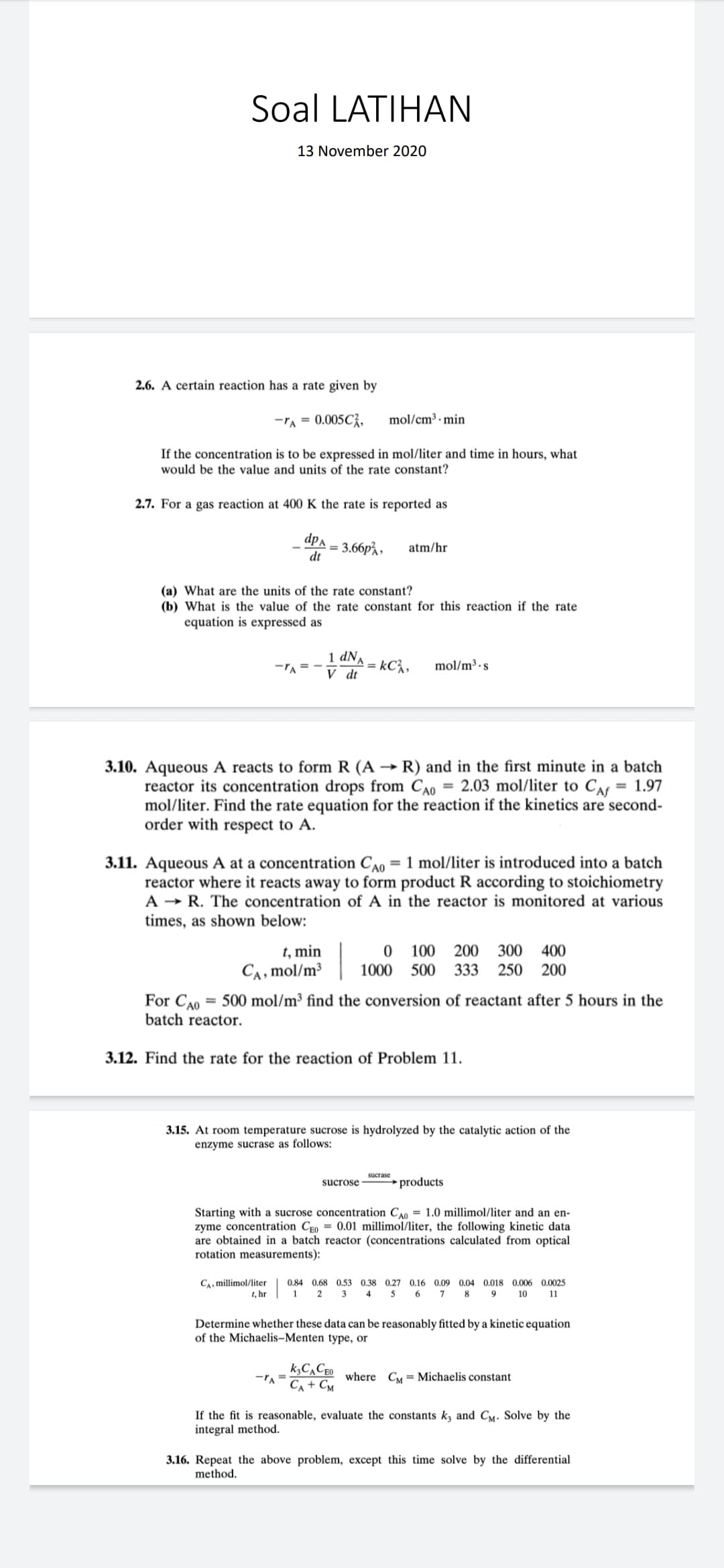
Transcribed Image Text:Soal LATIHAN
13 November 2020
2.6. A certain reaction has a rate given by
-TA = 0.005C;,
mol/cm³ · min
If the concentration is to be expressed in mol/liter and time in hours, what
would be the value and units of the rate constant?
2.7. For a gas reaction at 400 K the rate is reported as
_dpa
3.66p%,
dt
atm/hr
%3D
(a) What are the units of the rate constant?
(b) What is the value of the rate constant for this reaction if the rate
equation is expressed as
1 dNA
kC%,
V dt
-TA = -
mol/m³ · s
3.10. Aqueous A reacts to form R (A → R) and in the first minute in a batch
reactor its concentration drops from CA0 = 2.03 mol/liter to CAf
mol/liter. Find the rate equation for the reaction if the kinetics are second-
order with respect to A.
= 1.97
3.11. Aqueous A at a concentration CA0 = 1 mol/liter is introduced into a batch
reactor where it reacts away to form product R according to stoichiometry
A → R. The concentration of A in the reactor is monitored at various
times, as shown below:
%3D
t, min
CA, mol/m³
100 200
300
400
1000 500 333
250
200
For CA0 = 500 mol/m³ find the conversion of reactant after 5 hours in the
batch reactor.
3.12. Find the rate for the reaction of Problem 11.
3.15. At room temperature sucrose is hydrolyzed by the catalytic action of the
enzyme sucrase as follows:
sucrase
sucrose
products
Starting with a sucrose concentration C0 = 1.0 millimol/liter and an en-
zyme concentration Ceo = 0.01 millimol/liter, the following kinetic data
are obtained in a batch reactor (concentrations calculated from optical
rotation measurements):
CA, millimol/liter
t, hr
0.84 0.68 0.53 0.38 0.27 0.16 0.09 0.04 0.018 0.006 0.0025
1 2 3
4
5 6 7
8 9 10
11
Determine whether these data can be reasonably fitted by a kinetic equation
of the Michaelis–Menten type, or
k,C,CEO
Ca + Cm
where Cy = Michaelis constant
If the fit is reasonable, evaluate the constants k, and Cy. Solve by the
integral method.
3.16. Repeat the above problem, except this time solve by the differential
method.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The