Are the given reactions examples of general acid/base catalysis, covalent catalysis, catalysis by approximation (proximity effect), or metal-ion catalysis? Glu HN R' Enzyme substrate complex Glu H HN R' oe ***** Zn2+ Tetrahedral intermediate NMP kinases bring two nucleotides, such as AMP and ATP, together to facilitate the transfer of a phosphoryl group from one nucleotide to the other (creating two ADP). The enzyme positions the adenosine triphosphate (ATP) such that the gamma phosphate group is placed adjacently to the phosphate group of the NMP kinase. This facilitates the transfer of the phosphate between the two molecule. In aspartate ammonia lyase, a deprotonated serine sidechain performs a nucleophilic attack on a carbonyl carbon of the substrate. An active site histidine abstracts a proton from a substrate, making it more reactive, and then donates the proton back to the product. general acid/base catalysis catalysis by approximation metal-ion catalysis covalent catalysis
Are the given reactions examples of general acid/base catalysis, covalent catalysis, catalysis by approximation (proximity effect), or metal-ion catalysis? Glu HN R' Enzyme substrate complex Glu H HN R' oe ***** Zn2+ Tetrahedral intermediate NMP kinases bring two nucleotides, such as AMP and ATP, together to facilitate the transfer of a phosphoryl group from one nucleotide to the other (creating two ADP). The enzyme positions the adenosine triphosphate (ATP) such that the gamma phosphate group is placed adjacently to the phosphate group of the NMP kinase. This facilitates the transfer of the phosphate between the two molecule. In aspartate ammonia lyase, a deprotonated serine sidechain performs a nucleophilic attack on a carbonyl carbon of the substrate. An active site histidine abstracts a proton from a substrate, making it more reactive, and then donates the proton back to the product. general acid/base catalysis catalysis by approximation metal-ion catalysis covalent catalysis
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter23: Fatty Acid Catabolism
Section: Chapter Questions
Problem 21P: Using the ActiveModel for enoyl-CoA dehydratase, give an example of a case in which conserved...
Related questions
Question
100%
Q2:
The options to pick for each are:
- general acid/base catalysis
- catalysis by approximation
- metal-ion catalysis
- covalent catalysis
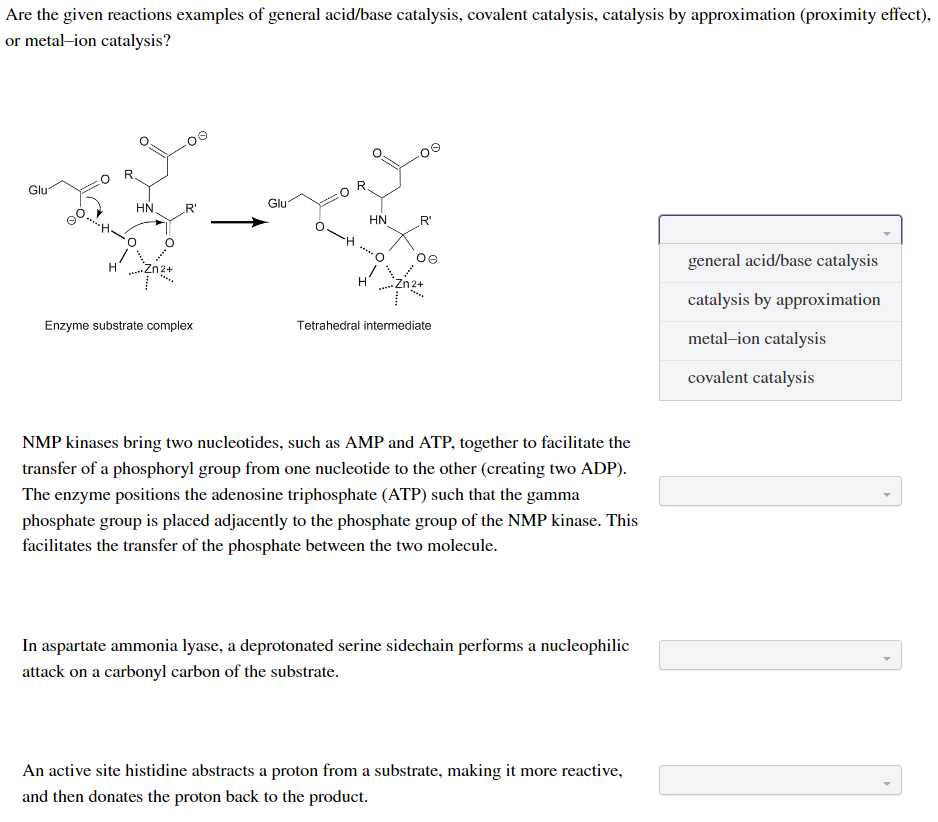
Transcribed Image Text:Are the given reactions examples of general acid/base catalysis, covalent catalysis, catalysis by approximation (proximity effect),
or metal-ion catalysis?
Glu
H
R.
HN.
R'
Enzyme substrate complex
Glu
R
H
HN
R'
Zn2+
PERE
Tetrahedral intermediate
NMP kinases bring two nucleotides, such as AMP and ATP, together to facilitate the
transfer of a phosphoryl group from one nucleotide to the other (creating two ADP).
The enzyme positions the adenosine triphosphate (ATP) such that the gamma
phosphate group is placed adjacently to the phosphate group of the NMP kinase. This
facilitates the transfer of the phosphate between the two molecule.
In aspartate ammonia lyase, a deprotonated serine sidechain performs a nucleophilic
attack on a carbonyl carbon of the substrate.
An active site histidine abstracts a proton from a substrate, making it more reactive,
and then donates the proton back to the product.
general acid/base catalysis
catalysis by approximation
metal-ion catalysis
covalent catalysis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning