As discussed in Chapters 25 and 26, a polymer is a very large molecule that contains many repeating units called monomers. The reaction here shows, for example, how styrene reacts to form polystyrene. The reaction is initiated by the electrophilic addition of H+ from an acid like sulfuric acid, which generates an initial carbocation. Afterward, that carbocation behaves as an electrophile in the presence of another molecule of styrene, resulting in yet another carbocation. This reaction can repeat many thousands of times to build up the polymer. With this in mind, draw the detailed mechanism that illustrates the initiation of the polymerization reaction and the addition of the first two monomers, as shown at the right. H,SO4 C6H5 CGH5 CgH5 Styrene Polystyrene H,SO4 CgH5 CGH5 C6H5
As discussed in Chapters 25 and 26, a polymer is a very large molecule that contains many repeating units called monomers. The reaction here shows, for example, how styrene reacts to form polystyrene. The reaction is initiated by the electrophilic addition of H+ from an acid like sulfuric acid, which generates an initial carbocation. Afterward, that carbocation behaves as an electrophile in the presence of another molecule of styrene, resulting in yet another carbocation. This reaction can repeat many thousands of times to build up the polymer. With this in mind, draw the detailed mechanism that illustrates the initiation of the polymerization reaction and the addition of the first two monomers, as shown at the right. H,SO4 C6H5 CGH5 CgH5 Styrene Polystyrene H,SO4 CgH5 CGH5 C6H5
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 51QAP
Related questions
Question
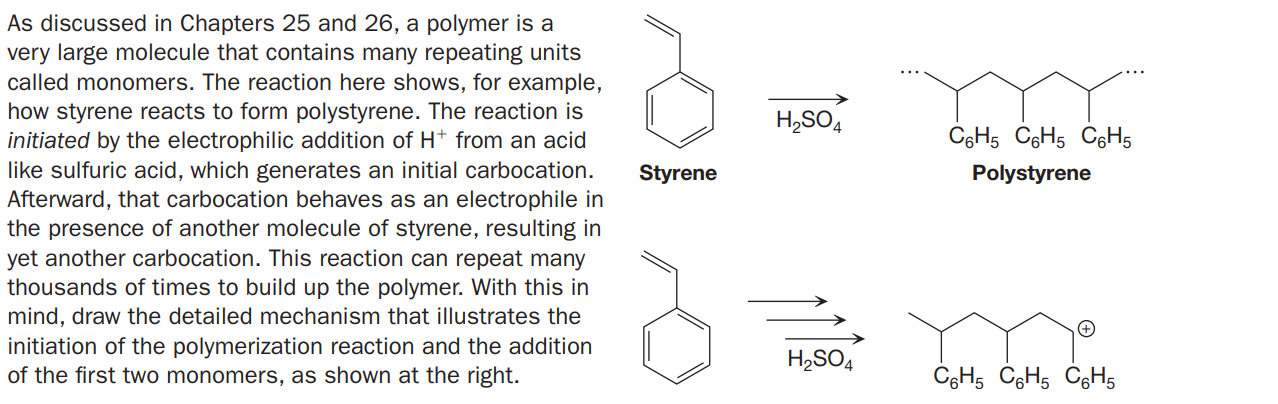
Transcribed Image Text:As discussed in Chapters 25 and 26, a polymer is a
very large molecule that contains many repeating units
called monomers. The reaction here shows, for example,
how styrene reacts to form polystyrene. The reaction is
initiated by the electrophilic addition of H+ from an acid
like sulfuric acid, which generates an initial carbocation.
Afterward, that carbocation behaves as an electrophile in
the presence of another molecule of styrene, resulting in
yet another carbocation. This reaction can repeat many
thousands of times to build up the polymer. With this in
mind, draw the detailed mechanism that illustrates the
initiation of the polymerization reaction and the addition
of the first two monomers, as shown at the right.
H,SO4
C6H5 CGH5 CgH5
Styrene
Polystyrene
H,SO4
CgH5 CGH5 C6H5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co