Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter7: Chemical Bonding And Molecular Geometry
Section: Chapter Questions
Problem 105E: Describe the molecular structure around the indicated atom or atoms: (a) the sulfur atom in sulfuric...
Related questions
Question
Answer in conversion factors
Transcribed Image Text:© CaH12O6. in g/mol - Google Sear
A Determine the number of atoms
Question 11 of 20
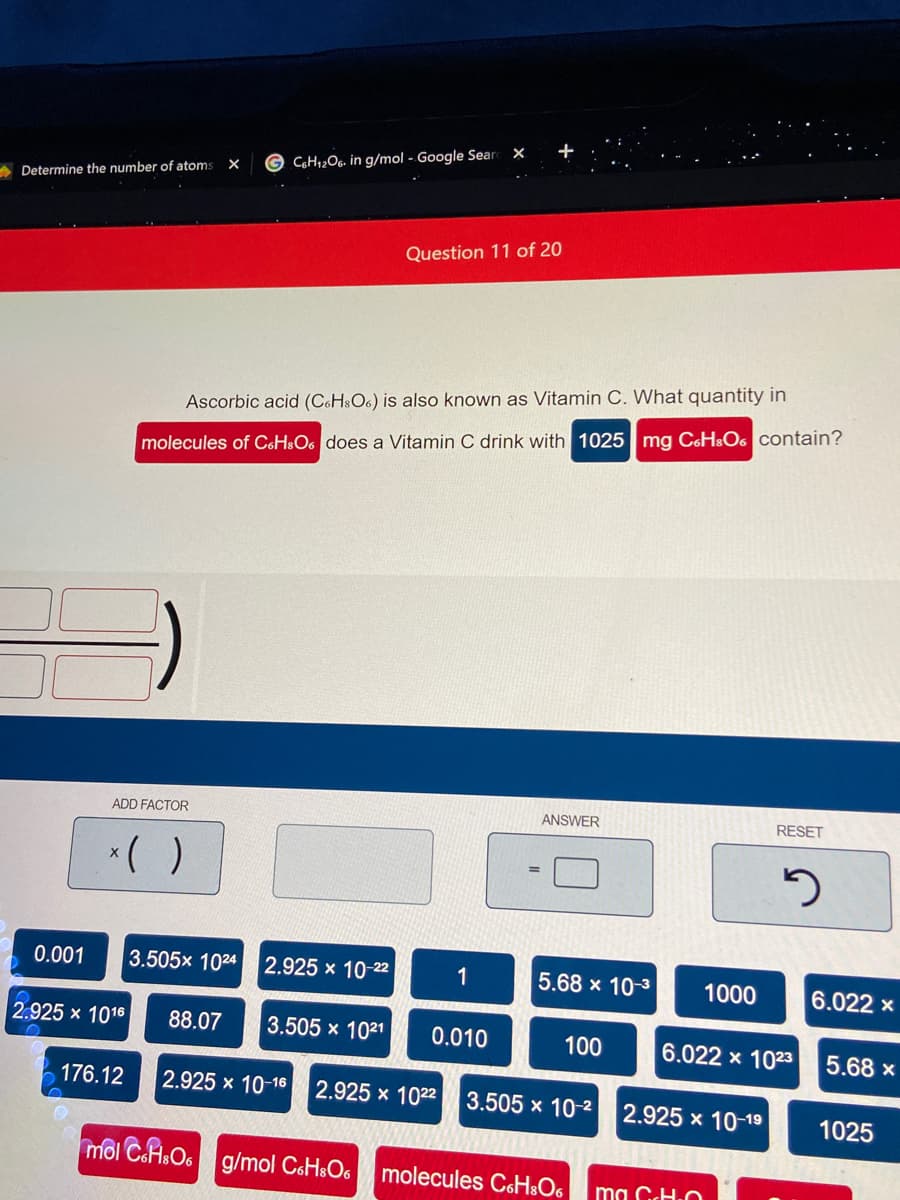
Ascorbic acid (CoHsO6) is also known as Vitamin C. What quantity in
molecules of C6H$O6 does a Vitamin C drink with 1025 mg C6HSO6 contain?
ADD FACTOR
ANSWER
RESET
*( )
0.001
3.505x 1024 2.925 x 10-22
1
5.68 x 10-3
1000
6.022 x
2.925 x 1016
88.07
3.505 x 1021
0.010
100
6.022 x 1023
5.68 x
176.12
2.925 x 10-16
2.925 x 1022
3.505 x 10-2
2.925 x 10-19
1025
môl CaAsO6 g/mol CoH&O6
molecules CoH&O6
ma GcH.O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning