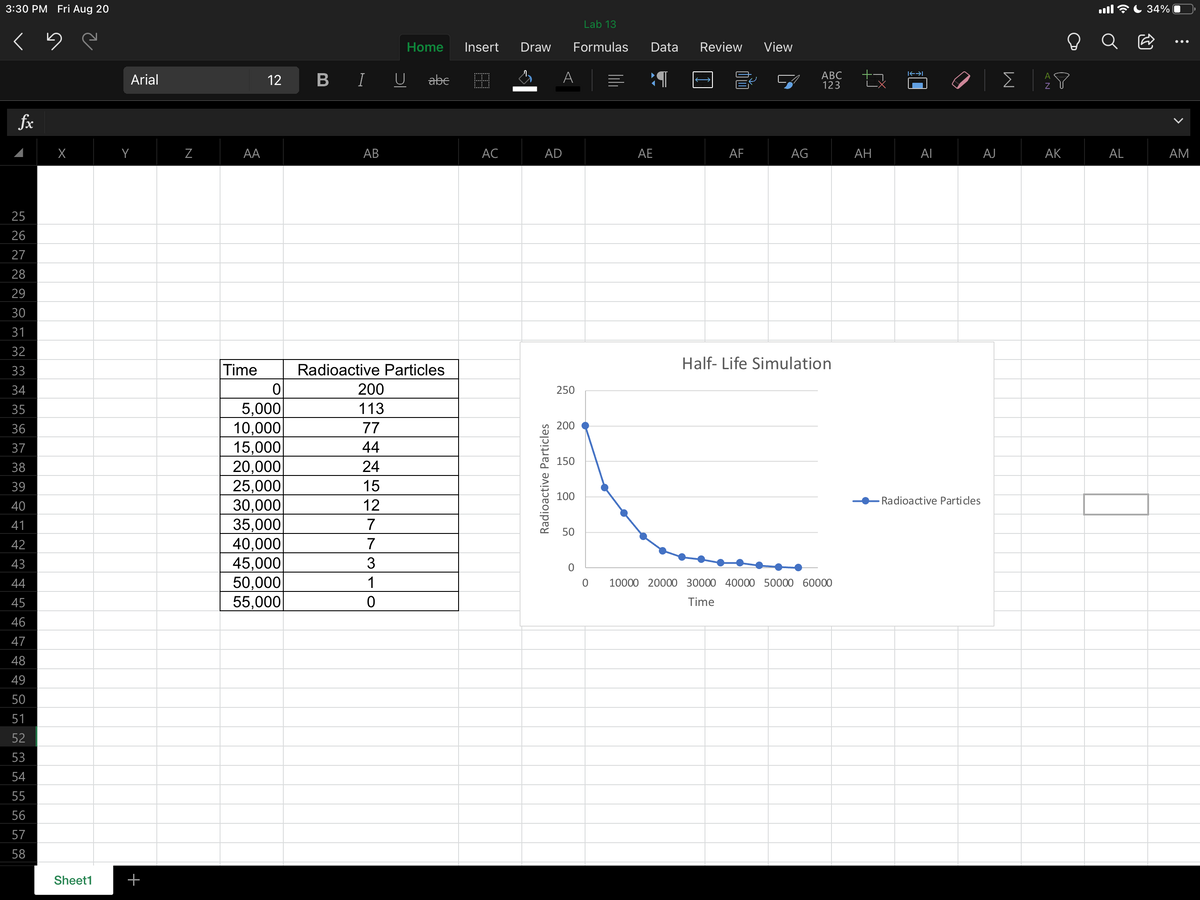
Assume that your "radioactivity" was C-14 (carbon-14, carbon with mass number 14) and that it undergoes beta decay. Write the balanced nuclear equation for this reaction.
Assume that your "radioactivity" was C-14 (carbon-14, carbon with mass number 14) and that it undergoes beta decay. Write the balanced nuclear equation for this reaction.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.6QAP
Related questions
Question
- Assume that your "radioactivity" was C-14 (carbon-14, carbon with mass number 14) and that it undergoes beta decay. Write the balanced
nuclear equation for this reaction.
Transcribed Image Text:3:30 PM Fri Aug 20
?C 34% O
Lab 13
Home
Insert
Draw
Formulas
Data
Review
View
Arial
12
B IU
abe
A
АВС
123
Σ
fx
Y
А
АВ
AC
AD
AE
AF
AG
АН
AI
AJ
AK
AL
AM
25
26
27
28
29
30
31
32
Half- Life Simulation
33
Time
Radioactive Particles
34
200
250
5,000|
10,000
15,000
20,000
25,000|
30,000
35,000
40,000
45,000
50,000|
55,000
35
113
36
77
200
37
44
150
38
24
39
15
100
Radioactive Particles
40
12
41
7
50
42
7
43
3
44
1
10000 20000 30000 40000 50000 60000
45
Time
46
47
48
49
50
51
52
53
54
55
56
57
58
Sheet1
lili
N
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
