At the end of the school year, a high school chemistry teacher asks one of the students to stay after school and help clean the storage room. While cleaning, the student finds a rectangular block of metal that is not labeled and is therefore unknown. a) The student wants to identify the metal using density. The student measures each side of the block (using a centimeter ruler) and determines the length is 3.00 cm, height is 5.00 cm, and width is 5.00 cm. The student then takes the mass of the block using an electronic balance and reports the mass as 337.50 g. What is the density of the rectangular block? Make sure to show all work, use significant figures, and end with a unit. b) The student then researches the density of different materials, as seen in the table below. What is the identity of the rectangular block?
At the end of the school year, a high school chemistry teacher asks one of the students to stay after school and help clean the storage room. While cleaning, the student finds a rectangular block of metal that is not labeled and is therefore unknown. a) The student wants to identify the metal using density. The student measures each side of the block (using a centimeter ruler) and determines the length is 3.00 cm, height is 5.00 cm, and width is 5.00 cm. The student then takes the mass of the block using an electronic balance and reports the mass as 337.50 g. What is the density of the rectangular block? Make sure to show all work, use significant figures, and end with a unit. b) The student then researches the density of different materials, as seen in the table below. What is the identity of the rectangular block?
University Physics Volume 1
18th Edition
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:William Moebs, Samuel J. Ling, Jeff Sanny
Chapter1: Units And Measurement
Section: Chapter Questions
Problem 1.7CYU: Check Your Understanding Figure 1.4 says the mass of the atmosphere is 1019kg . Assuming the density...
Related questions
Question

Transcribed Image Text:At the end of the school year, a high school chemistry teacher asks one of the
students to stay after school and help clean the storage room. While cleaning, the
student finds a rectangular block of metal that is not labeled and is therefore
unknown.
a) The student wants to identify the metal using density. The student measures each
side of the block (using a centimeter ruler) and determines the length is 3.00 cm,
height is 5.00 cm, and width is 5.00 cm. The student then takes the mass of the
block using an electronic balance and reports the mass as 337.50 g. What is the
density of the rectangular block? Make sure to show all work, use significant figures,
and end with a unit.
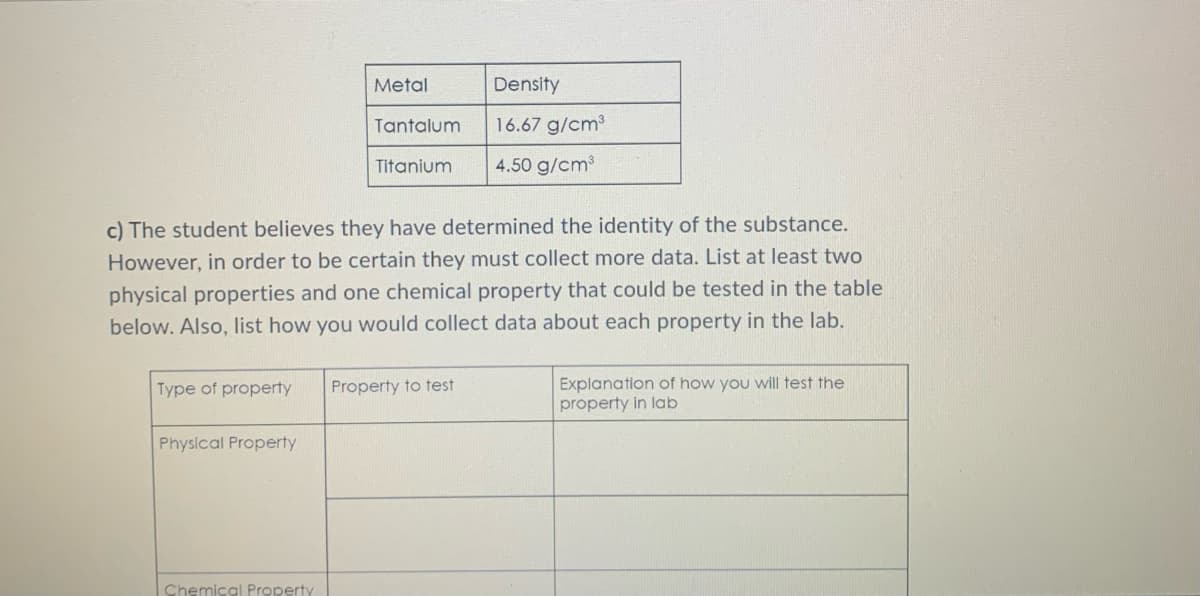
b) The student then researches the density of different materials, as seen in the table
below. What is the identity of the rectangular block?
Metal
Density
Tantalum
16.67 g/cm³
Transcribed Image Text:Metal
Density
Tantalum
16.67 g/cm³
Titanium
4.50 g/cm³
c) The student believes they have determined the identity of the substance.
However, in order to be certain they must collect more data. List at least two
physical properties and one chemical property that could be tested in the table
below. Also, list how you would collect data about each property in the lab.
Type of property Property to test
Explanation of how you will test the
property in lab
Physical Property
Chemical Property
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning