ATOM Zinc (Zn) Lithium (Li) Lead (Pb) Platinum For which component of the battery is this element used? anode What visual evidence What chemical equation describes the Translate the chemical equation. suggests chemical change is happening to the individual chemical change What's happening to each atom in the chemical change process? atoms? happening to the atoms? Zn --> Zn2+ +2e- oxidation. Zinc gains a 2+ charge and Silver gains a neutral charge. What's the label assigned to this type of chemical change? The label assigned to this type of chemical change
ATOM Zinc (Zn) Lithium (Li) Lead (Pb) Platinum For which component of the battery is this element used? anode What visual evidence What chemical equation describes the Translate the chemical equation. suggests chemical change is happening to the individual chemical change What's happening to each atom in the chemical change process? atoms? happening to the atoms? Zn --> Zn2+ +2e- oxidation. Zinc gains a 2+ charge and Silver gains a neutral charge. What's the label assigned to this type of chemical change? The label assigned to this type of chemical change
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter8: The Periodic Table: Structure And Trends
Section: Chapter Questions
Problem 8.83QE
Related questions
Question
100%
Redox battery sim link: https://interactives.ck12.org/simulations/chemistry/redox-reaction/app/index.html??screen=sandbox
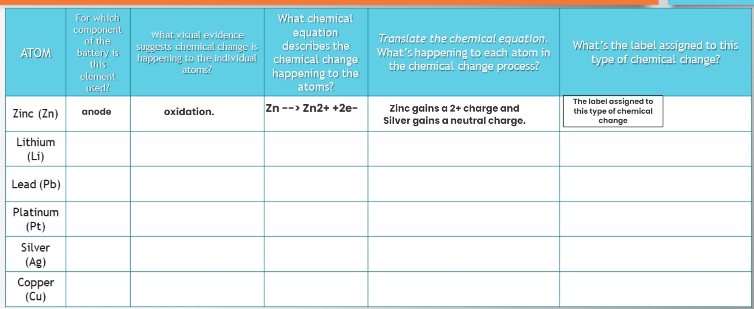
Transcribed Image Text:ATOM
Zinc (Zn)
Lithium
(Li)
Lead (Pb)
Platinum
(Pt)
Silver
(Ag)
Copper
(Cu)
For which
component
of the
battery is
this
element
used?
anode
What visual evidence
suggests chemical change is
happening to the individual
atoms?
oxidation.
What chemical
equation
describes the
chemical change
happening to the
atoms?
Zn --> Zn2+ +2e-
Translate the chemical equation.
what's happening to each atom in
the chemical change process?
zinc gains a 2+ charge and
Silver gains a neutral charge.
What's the label assigned to this
type of chemical change?
The label assigned to
this type of chemical
change
Expert Solution
Step 1
| ATOM | What evidence suggests chemical changes is happening to the individual atom? | What chemical equation describe the chemical change happening to the atoms? | Translate the chemical equation, what's happening to each atom in the chemical change process? | What's the label assign to this type of chemical change? | |
| Zinc (Zn) | Anode | Oxidation | Zn ⟶ Zn2+ + 2e- | Zinc gains a 2+ charge | The label assign to this type of chemical change is oxidation |
| Lithium (Li) | Anode | Oxidation | Li ⟶ Li+ + e- | Lithium gains a 1+ charge | The label assign to this type of chemical change is oxidation |
| Lead (Pb) | Anode | Oxidation | Pb ⟶ Pb2+ + 2e- | Lead gains a 2+ charge | The label assign to this type of chemical change is oxidation |
| Platinum (Pt) | Cathode | Reduction | Pt2+ + 2e- ⟶ Pt | Platinum gains neutral charge | The label assign to this type of chemical change is reduction |
| Silver (Ag) | Cathode | Reduction | Ag+ + e- ⟶ Ag | Silver gains neutral charge | The label assign to this type of chemical change is reduction |
| Copper (Cu) | Cathode | Reduction | Cu2+ + 2e- ⟶ Cu | Copper gains neutral charge | The label assign to this type of chemical change is reduction |
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning