Attempt 3 NH, is a weak base (K, = 1.8 x 10) and so the salt NH,Cl acts as a weak acid. What is the pH of a solution that is 0.038 M in NH,Cl at 25 °C? pH =
Q: The hydronium ion concentration of an aqueous solution of 0.422 M diethylamine (a weak base with the…
A:
Q: Pre-lab question #3-3: Consider the following equilibrium for nitrous acid, HNO2, a weak acid: HNO2…
A: In this question, we will see the equilibrium shifting after addition of HCl on Equilibrium…
Q: EXAMPLE 9-8 Calculate the hydronium ion concentration in a solution that is 2.0 X 10-4 M in aniline…
A: The Equilibrium Reaction of hydrolysis of C6H5NH3+ given in the question is - C6H5NH3+ + H2O…
Q: What is [H30+] if I have a 0.1 M NaOH? This is the correct answer but i don't know how it was…
A: Answer:- This question is answered by using the simple concept of calculation of concentration of…
Q: II. Show complete solution for the following problems. Box your final answer. 1. If the H+…
A: 1. Given that: [H+] = 0.00001M H2O(l) <.....> H+(aq) + OH-(aq)
Q: Calculate the Kb of an aqueous (25 oC) Na2SO4 solution. Ka HSO4– = 1.21 x 10–2.
A: Given :- Ka = 1.21×10-2 Kb = ?
Q: QUESTION 6 Calculate the pH of a solution that contains 3.9 x 10 MH30* at 25°C, 4.41 8.59 5.41 0.59…
A: Given, A solution contain concentration of [H3O+] is 3.9×10 -5 , the pH of the solution is:
Q: Problem Solving. Solve the following problems, use GRESA format in answering A 0.0001 molar basic…
A:
Q: 1.80
A: Dear student I have given answer to your question in the image format.
Q: Part A What is the H+ concentration for an aqueous solution with pOH= 4.47 at 25 °C? Express your…
A: Concentration of H+ can be calculated as follows
Q: What is the pH of a 0.298 M C6H5CO2H M solution if the Ka of C6H5CO2H is 6.5 x 10-5? Your Answer:…
A: Given Concentration of C6H5COOH = 0.298 Ka for C6H5COOH = 6.5×10-5
Q: B: What is the pH of 0.6 M NH,Cl solution? Discuss your results. (K of NH= 1.8 x 10 NH4+ + H2O+→…
A: Firstly, write the equilibrium constant for the given reaction. It is calculated by dividing the…
Q: acid has a pH of 2.50 (show work for calculations). Determine the [H3O+] concentration. Determine…
A: Given, pH = 2.50 pH = -log([H3O+]) 2.50 = -log([H3O+)] [H3O+] = 0.00316 M [OH-] = Kw/[H+] =…
Q: (a) The pH of a solution is 5.42 + 0.05. Find [H1] and its uncertainty. (b) What is the relative…
A: The solution considered has a pH of 5.42 + 0.05 where 0.05 is the uncertainty in pH value.
Q: K, value for H2S is 7.00. What mole ratio of KHS to H2S is needed to prepare a pH of 6.62? Submit…
A: The question is based on the concept of buffer solution. A buffer is a solution which resist any…
Q: Lab Report #2-2-1: 0.1 M Na2CO3 solution, using the given pH data, calculate: [H*], [OH"] O [H*] =…
A:
Q: Pre-lab question #3-4: Consider the following equilibrium for nitrous acid, HNO2, a weak acid: HNO2…
A: Given reaction is HNO2 + H2O <-> H3O+ + NO2-
Q: Lab Report #1-2: 0.1 M NaCl solution, using the given pH data, calculate: [H*] M, [OH`] M O [H*]…
A: Explanation to the correct answer is given below
Q: The value of K for nitrous acid is 4.50x104. What is the value of K, for its conjugate base, NO2?…
A: The product of acid- dissociation constant for acid and the base dissociation constant for it…
Q: Hello If we have a solution of 1L with 0,10M of acetic acid (Ka = 1,8x10-5)and 0,10M of sodium…
A: When a weak acid and its salt is present in solution they form a buffer solution and pH of solution…
Q: The pH of an aqueous solution at 25°C was found to be 3.90. The pOH of this solution is The…
A: pH is defined as the negative logarithm of hydrogen or hydronium ion concentration present in the…
Q: What is the pH of a 4.0×10 M solution of KOH at 25 °C? Submit Show Approach Show Tutor Steps
A:
Q: B: What is the pH of 0.6 M NH.CI solution? Discuss your results. (K of NH= 1.8 x 105) NH. + H2O+ H3O…
A:
Q: I am getting the wrong amount of sig figs for each equation, even though I am using the smallest…
A: The mass of 1 mole of viruses is calculated as shown below,
Q: What is the pH of a 0.151 M aqueous solution of sodium cyanide, NaCN at 25 °C? (K, for HCN =…
A: For the reaction,
Q: Ka for phenol (a weak acid), C6H5OH, is 1.60×10-10 Ka for nitrous acid, HNO₂, is 4.00×10-4. Ka for…
A: We know ; Strong acid ⇌ H+ + conjugate base (weak)
Q: PARTICIPATION op Which acid is stronger: HF (Ka=6.4x10“ ) or NH,*(Kb for NH, is 1.8x10*)?
A: Larger the Ka value, stronger will be the acid.
Q: Problem Solving. Show all pertinent computations. Calculate the pH of a solution containing 0.75 M…
A: The solution contains a mixture of 0.75 M lactic acid and 0.25 M sodium lactate. The acidity…
Q: Calculate the pH of a 0.52 M NH4Cl solution. (Kb for NH3 = 1.8 x 10-5) (Need to show your work…
A: Dissociation of 0.52 M NH4Cl solution is 0.52 M NH4Cl ⇌ 0.52 M NH4++0.52 M Cl- The ICE table is as…
Q: PARTICIPATION openstax What is the [H,O*) of 0.10 M propanoic acid (CH,CH,COOH, can simplify to HPr)…
A: It's a multiple question type. Given information, (1) [Propanoic acid] = 0.10 M Ka for propanoic…
Q: Example 2-16 Calculate the p-value for each ion in a solution that is 2.00 x 10-3 M in NaCl and 5.4…
A: Concentration of NaCl = 2 × 10-3 M Concentration of HCl = 5.4 × 10-4 M P value For each ion…
Q: Consider two acids: Cl,CHCO,H (dichloroacetic acid, pK,=1.3) and HCO,H (formic acid, pK, = 3.8).…
A: In this question, we will select the strongest acid. You can see details explanation and answer…
Q: Question attached
A: According to Henderson Hasselbalch equation, pH = pKa+log[A-][HA] where pKa is the negative…
Q: Homework Q1 :- What is the pK, of acetic acid, if K, for acetic acid is 1.58 x 10-6?
A: Pka is equal to negative logarithm of ka
Q: [References) The pH of a 0.179 M solution of a weak acid, HB, is 2.57. What is K, for the weak acid?…
A: 1- First calculate the H+ ion concentration in the solution. pH = -log([H+]) -pH = log([H+])…
Q: Show work, show correct significant figures, label units 1. Find pH for the following: a. [H,O*]=…
A: PH is defined as negative logarithm of concentration of H+ ions. Given, [H3O+] = 5.7 x 10-3 M [OH-]…
Q: Pre-lab question #3-1: Consider the following equilibrium for nitrous acid, HNO2, a weak acid: HNO2…
A:
Q: Pre-lab question #3-2: Consider the following equilibrium for nitrous acid, HNO2, a weak acid: HNO2…
A: When there is a balance between direct and reverse reaction, chemical equilibrium is achieved. The…
Q: Calculate the pH of a 0.471 M aqueous solution of diethylamine ((C₂H5)₂NH, Kb = 6.9x10-4). pH =…
A:
Q: What is the pH of a solution that contains 0.43 M CH3COOH and 0.32 M CH;COONa at 25°C (Ka=1.8 x 10)?…
A:
Q: The pKa of MOPS is 7.20. Draw the equilibrium involved in the dissociation of the proton. 1. Suppose…
A: Hi, as you have posted multiple questions and have not mentioned which question you want us to solve…
Q: What is the enthalpy of solution of NaOH in kJ/mol? AHF[N2OH (s)] = -425.6 kJ/mol AH [NAOH (aq)] =…
A: Given, ∆Hf°[NaOH(s)]= -425.6 kJmol∆Hf°[NaOH(aq)]=-470.1kJmol
Q: ns 13.1-13.3) NEW [References) What is the pH of a solution obtained by adding 158 mL of 0.206 M HCl…
A: Volume of HCL= 158 ml Concentration of HCL =0.206M Volume of HNO3 =926 ml pH of HNO3 =2.550
Q: A 0.068 M solution of an unknown weak acid has a pH = 1.86. What is the Ka of the acid?
A:
Q: What is the concentration of hydroxide ion in a 0.170 M aqueous solution of hydroxylamine, NH2OH?…
A: pH of solution is defined as negative logarithm of hydrogen ions concentration.
Q: Question attached
A: Given data, The number of moles of HCl (n) = 6.20 × 10-3 Volume = 1 L Number of moles = molarity To…
Q: Lab Report #5-2-3: 0.1 M NaHCO3 solution, using the given pH data, write expression for equilibrium…
A: The question is based on the concept of salt hydrolysis. We have to write equilibrium constant…
Q: A 0.345 M of generic weak acid was prepared and has a Kb of 3.91 x 10-10. What is the pH of this…
A:
Q: Assignment 1. Consider the acid-base reaction: H,CO,º → HCO; + H+ for which K = 10-6.35, Which way…
A:
Q: 3. By definition the pH of a dilute solution of a weak acid equals its pKa only when the…
A:

Dissociation constant of base is given by
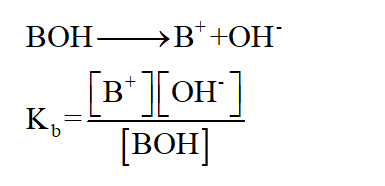
The Ka is calculated as
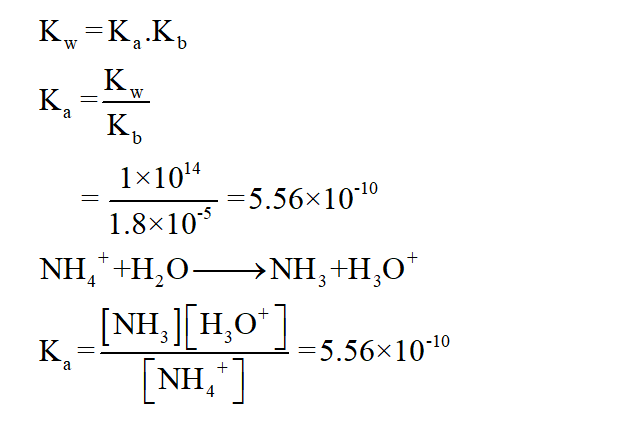
Step by step
Solved in 4 steps with 4 images

- You start with a 10.0 mM solution of H3PO4 at a pH of 7.0. If you were to add 2.0 mL of 100 mM HCl what would the resulting pH be? Please show work.A solution of 0.80 M acetic acid acid has a pH of 2.50 (show work for calculations). Determine the [H3O+] concentration. Determine the [OH-] concentration. Deterimine the pH of the acetic acid if it is a strong acid. Based on the given pH and the one calculated if acetic acid is a strong acid, is acetic acid a weak acid or a strong acid? Explain. The formula of benzoic acid is CH3COOH, write out the Ka equation.A sample of rainwater is observed to have a pH of 7.4. If only atmospheric CO2 at 380 ppm and limestone dust are present in the atmosphere to alter the pH from a neutral value, and if each raindrop has a volume of 0.02 cm^3, what mass of calcium is present in each raindrop? please answer fast i give upvote
- please someone help with explanation thank you A 0.200 M solution of an acid, HBrun, has a pH = 1.00. What is the value of the ionization constant, Ka, for this acid? a. 0.200 b. 0.0400 c. 1.00 x 103 d. 1.00 e. 1.00 x 101Answer the following, include your work and reasoning 1)A certain weak base has a Kb of 7.10×10-7. What initial concentration of this base will produce a pH of 10.02 at equilibrium? 2)For a 1.0×10-2 M solution of CH3NH3Br(aq) at equilibrium, arrange the following species by their relative molar amounts in solution, largest amount first. 3)CH3NH2 is a weak base (?b = 5.0×10-4), so the salt CH3NH3NO3 acts as a weak acid. What is the pH of a solution that is 0.0450M CH3NH3NO3 at 25oC?Hydroxylamine, HONH2, readily forms salts such as hydroxylamine hydrochloride which are used as antioxidants in soaps. Hydroxylamine has Kb of 9.1 × 10–9. What is the pH of a 0.054 M HONH2 solution? Please report 2 decimal places.
- What is [H+] with uncertainty for a solution of pH 7.5 ± 0.2? Thank you!A 2.5 M solution of a weak acid is prepared. Using a pH meter, the pH is measured to be 5.1. Calculate the acid ionization constant, Ka, of this weak acid. Show your work.1. Which of the following is the well known Henderson-Halsselbalch equation?. Group of answer choices A, pH = pKa+ log ([A-]/[HA]) B, pH = pKb+ log ([A-]/[HA]) C, pOH = pKa+ log ([A-]/[HA])



