Avg. Moisture Avg. Moisture Time, h Content, % (dry basis) Time, h Content, % (dry basis) 127 9. 41.8 112 10 38.5 96.8 12 30.8 83.5 14 26.4 4 73.6 16 20.9 64.9 18 16.5 6. 57.2 20 14.3 51.7 22 12.1 46.1 6.6
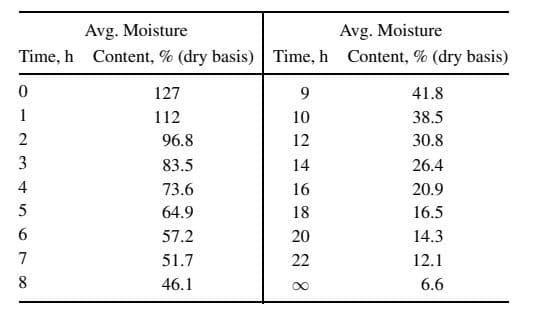
Gilliland and Sherwood obtained data for the drying of a water-wet piece of hemlock wood measuring 15.15x14.8x0.75 cm, where only the two largest faces were exposed to drying air, which was at 25oC and passed over the faces at 3.7 m/s. The wetbulb temperature of the air was 17oC and pressure was 1 atm. The data below were obtained for average moisture content (dry basis) of the wood as a function of time. From these data, determine whether Case 1 or Case 2 for the diffusion of moisture in solids applies. If Case 1 is chosen, determine the effective diffusivity; if Case 2, determine: (a) drying rate in g/h-cm2 for the constant-rate period, assuming a wood density of 0.5 g/cm3 (dry basis) and no shrinkage upon drying; (b) critical moisture content; (c) predicted parabolic moisture-content profile at the beginning of the fallingrate period; (d) effective diffusivity during the falling-rate period. In addition, for either case, describe what else could be determined from the data and explain how it could be verified
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 13 images









