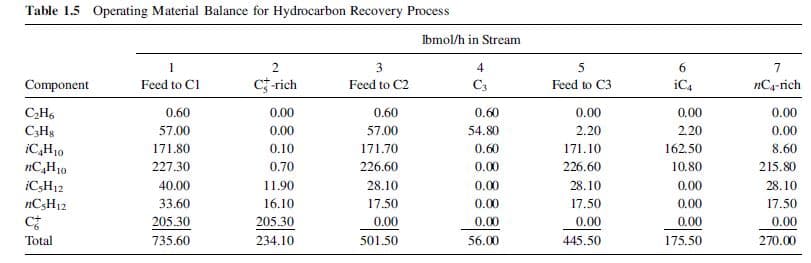
Table 1.5 Operating Material Balance for Hydrocarbon Recovery Process Ibmol/h in Stream 1 Feed to Cl 2. Component Feed to C2 Feed to C3 c-rich C3 iC4 nC4-rich CH6 C;H3 iC,H10 ИС,Но iC,H12 nC3H12 0.60 57.00 171.80 227.30 40.00 33.60 205.30 0.60 54.80 0.60 57.00 171.70 226.60 28.10 17.50 0.00 2.20 0.00 0.00 0.00 8.60 215.80 0.00 2.20 162.50 10.80 0.60 0.10 0.70 0.00 0.00 0.00 0.00 171.10 226.60 28.10 17.50 0.00 445.50 11.90 0.00 28.10 17.50 0.00 270.00 0.00 16.10 205.30 234.10 0.00 Total 735.60 0.00 501.50 56.00 175.50
Nitrogen injection to recover natural gas.
Nitrogen is injected into oil wells to increase the recovery of crude oil (enhanced oil recovery). It mixes with the natural gas that is produced along with the oil. The nitrogen must then be separated from the natural gas. A total of 170,000 SCFH (based on 60oF and 14.7 psia) of natural gas containing 18% N2, 75% CH4, and 7% C2H6 at 100oF and 800 psia is to be processed so that it contains less than 3 mol% nitrogen in a two-step process:
(1) membrane separation with a nonporous glassy polyimide membrane, followed by (2) pressure-swing adsorption using molecular sieves highly selective for N2( SPN2;CH4= 16) and completely impermeable to ethane. The pressure-swing adsorption step selectively adsorbs methane, giving 97% pure methane in the adsorbate, with an 85% recovery of CH4 fed to the adsorber. The non-permeate (retentate) gas from the membrane step and adsorbate from the pressure-swing adsorption step are combined to give a methane stream that contains 3.0 mol% N2. The pressure drop
across the membrane is 760 psi. The permeate at 20oF is compressed
to 275 psia and cooled to 100o F before entering the adsorption step. The adsorbate, which exits the adsorber during regeneration at 100oF and 15 psia, is compressed to 800 psia and cooled to 100oF before being combined with non-permeate gas to give the final pipeline natural gas.
(a) Draw a flow diagram of the process using appropriate symbols.Include compressors and heat exchangers. Label the diagram with the data given and number all streams.
(b) Compute component flow rates of N2, CH4, and C2H6 in lbmol/h
in all streams and create a material-balance table similar to Table 1.5.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images









