Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.8QAP
Related questions
Question
Please help with Question 1 (b)
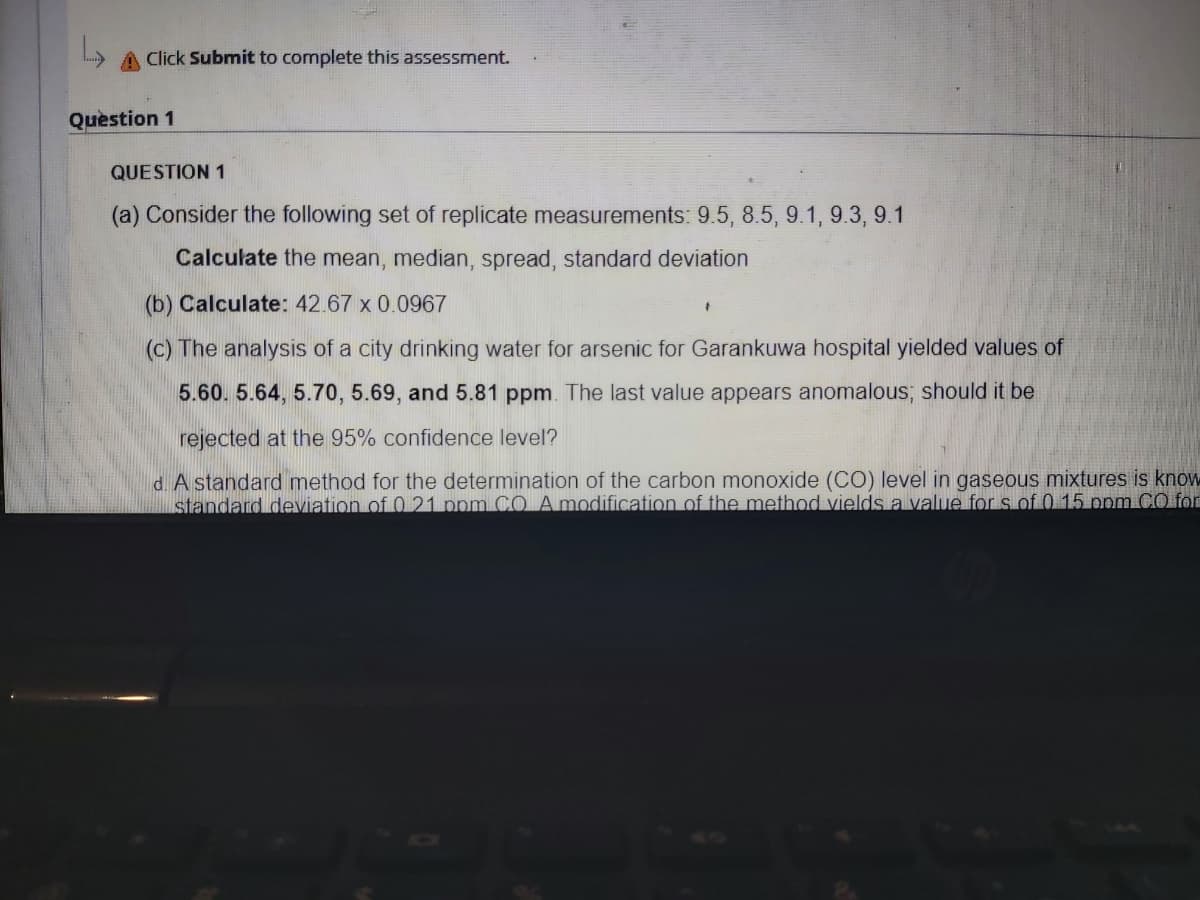
Transcribed Image Text:A Click Submit to complete this assessment.
Question 1
QUESTION 1
(a) Consider the following set of replicate measurements: 9.5, 8.5, 9.1, 9.3, 9.1
Calculate the mean, median, spread, standard deviation
(b) Calculate: 42.67 x 0.0967
(c) The analysis of a city drinking water for arsenic for Garankuwa hospital yielded values of
5.60. 5.64, 5.70, 5.69, and 5.81 ppm. The last value appears anomalous; should it be
rejected at the 95% confidence level?
d. A standard method for the determination of the carbon monoxide (CO) level in gaseous mixtures is know
standard deviation of 0 21 ppm CO A modification of the method vields a value for s of 0 15 pom CO for
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
