(b) How do the structures of these molecules account for these differences in solubility? Provide a full account of the structures and their influence on intermolecular forces that might account for the differences in this physical property. ( Name Solubility20 1-butanol 73 g/L butanal 7.6 g/100 mL butanone 27.5 g/100 mL 1-chlorobutane 0.5 g/L
(b) How do the structures of these molecules account for these differences in solubility? Provide a full account of the structures and their influence on intermolecular forces that might account for the differences in this physical property. ( Name Solubility20 1-butanol 73 g/L butanal 7.6 g/100 mL butanone 27.5 g/100 mL 1-chlorobutane 0.5 g/L
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 4RQ
Related questions
Question
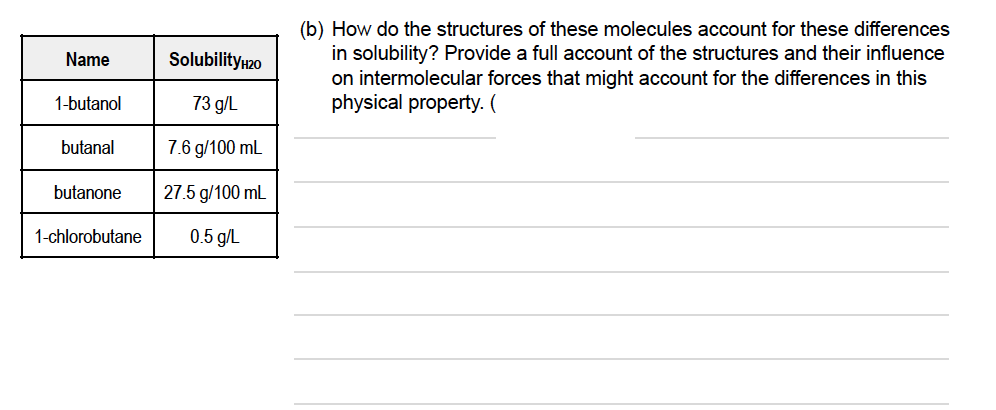
Transcribed Image Text:(b) How do the structures of these molecules account for these differences
in solubility? Provide a full account of the structures and their influence
on intermolecular forces that might account for the differences in this
physical property. (
Name
Solubility 20
1-butanol
73 g/L
butanal
7.6 g/100 mL
butanone
27.5 g/100 mL
1-chlorobutane
0.5 g/L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning