Balance the following equations using the change in oxidation number method. 1. K2Cr20, + FeCl2 + HCI – CrCl3 + KCI + FeCl, + H20 Substance oxidized: Substance reduced: Balanced equation:
Balance the following equations using the change in oxidation number method. 1. K2Cr20, + FeCl2 + HCI – CrCl3 + KCI + FeCl, + H20 Substance oxidized: Substance reduced: Balanced equation:
Related questions
Question
Please follow the steps in the other picture
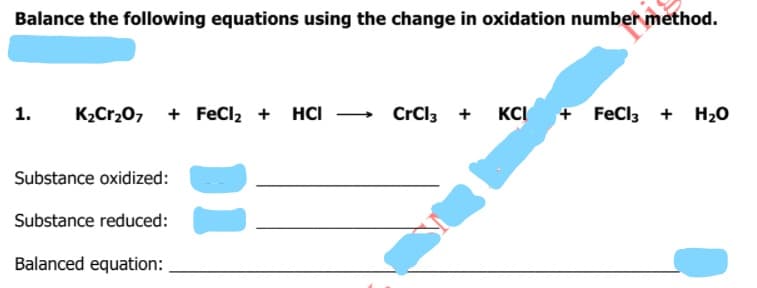
Transcribed Image Text:Balance the following equations using the change in oxidation number method.
1.
K2Cr20, + FeCl, + HCI - CrCl3 +
KCI
+ FeCl3 + H20
Substance oxidized:
Substance reduced:
Balanced equation:
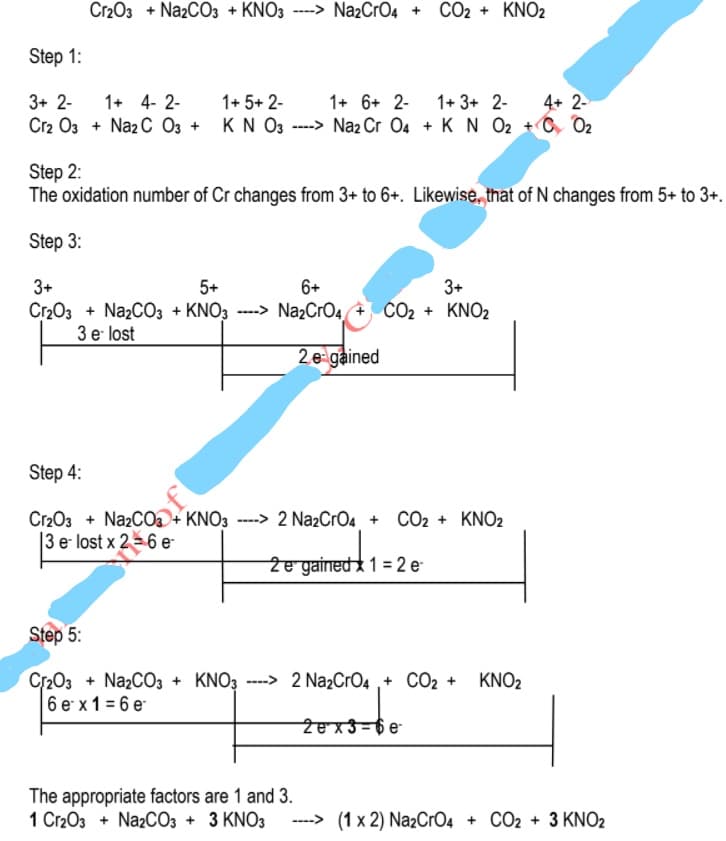
Transcribed Image Text:Cr203 + NazCO3 + KNO3 ----> Na2CrO4 + CO2 + KNO2
Step 1:
3+ 2-
1+ 4- 2-
1+ 5+ 2-
1+ 6+ 2-
1+ 3+ 2-
4+ 2-
Cr2 O3 + Naz C 03 + KN O3 ---> Na2 Cr 04 + K N O2 +C O2
Step 2:
The oxidation number of Cr changes from 3+ to 6+. Likewise, that of N changes from 5+ to 3+.
Step 3:
3+
Cr2O3 + NazCO3 + KNO3 ---> NazCrO4 + CO2 + KNO2
3 e lost
5+
6+
3+
2.e gained
Step 4:
Cr2O3 + NazCOa+ KNO3 ---> 2 Na2CrO4_ + CO2 + KNO2
|3 e lost x 26 e
Ze gained 1 = 2 e
Step 5:
Cr2O3 + NazC03 + KNO3 ----> 2 Na2CrO4 ,+ CO2 +
6 e x 1 = 6 e
KNO2
Zex3-6e
The appropriate factors are 1 and 3.
1 Cr203 + NazCO3 + 3 KNO3
----> (1 x 2) Na2CrO4 + CO2 + 3 KNO2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.