Bananas are somewhat radioactive due to the presence of substantial amounts of potassium. Potassium-40 decays by two different paths: 40 K Ca+B (89.3%) 19 20 40 , K2 40 KA+B* (10.7%) 19 18 The half-life for potassium decay is 1.3x10° years. Calculate the overall and the rate constants for the individual branch reactions.
Bananas are somewhat radioactive due to the presence of substantial amounts of potassium. Potassium-40 decays by two different paths: 40 K Ca+B (89.3%) 19 20 40 , K2 40 KA+B* (10.7%) 19 18 The half-life for potassium decay is 1.3x10° years. Calculate the overall and the rate constants for the individual branch reactions.
Related questions
Question
100%
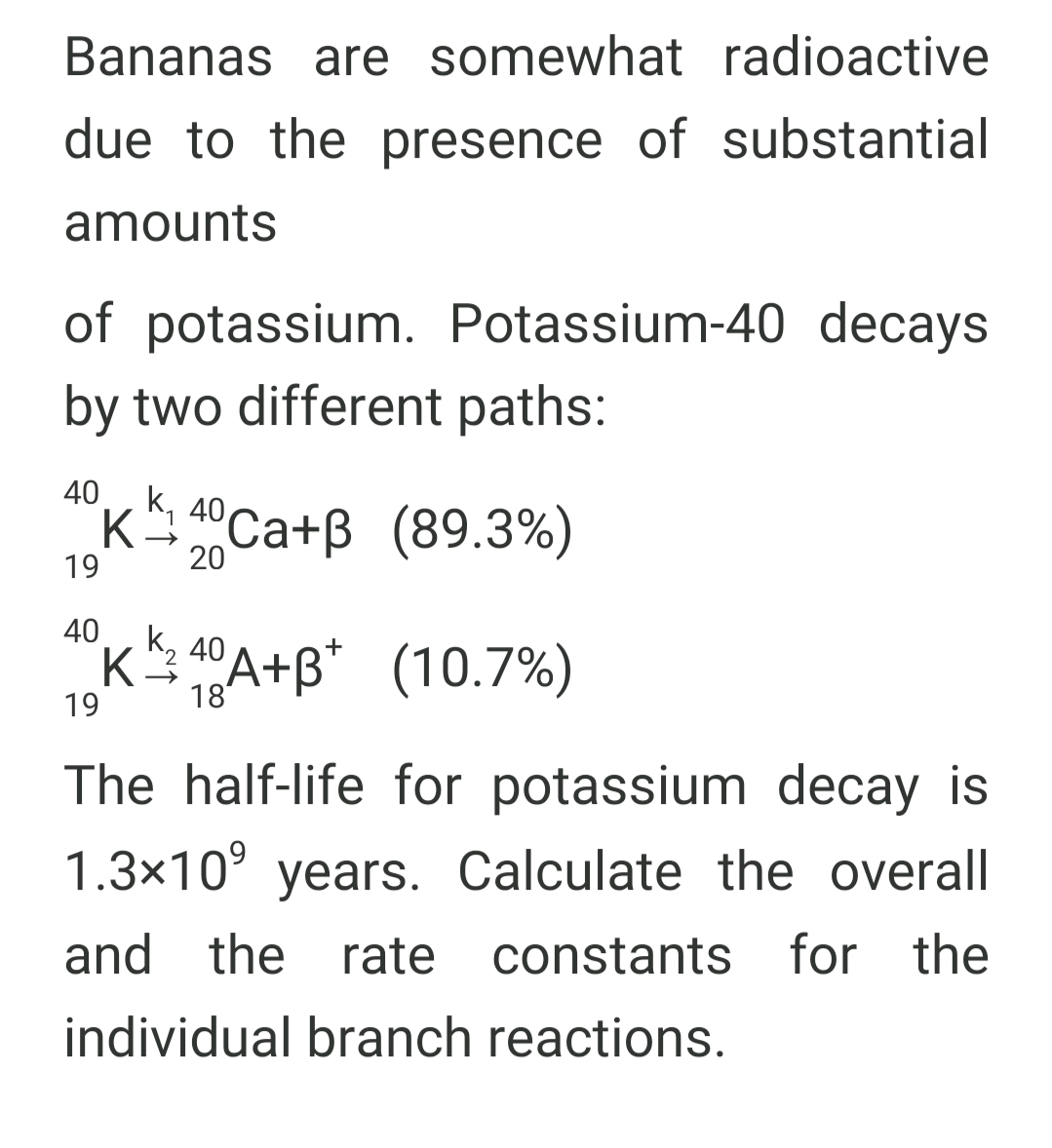
Transcribed Image Text:Bananas are somewhat radioactive
due to the presence of substantial
amounts
of potassium. Potassium-40 decays
by two different paths:
40
K 40Ca+B (89.3%)
1
19
20
40K k, 40 A+B* (10.7%)
19
18
The half-life for potassium decay is
1.3x10° years. Calculate the overall
and the rate constants for the
individual branch reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
