Based on the chemical equation, HCl(ag) + H2°(1) H30* (aq) + cl (aq) which of the following statement is correct? cI (ag) is the acid, H2O) is its conjugate base |(aq) is the acid, HCl(ag) is its conjugate base HC\(aq) is the acid, H3O* (ag) is its conjugate base HC(aq) is the acid, Cl" (ag) is its conjugate base
Based on the chemical equation, HCl(ag) + H2°(1) H30* (aq) + cl (aq) which of the following statement is correct? cI (ag) is the acid, H2O) is its conjugate base |(aq) is the acid, HCl(ag) is its conjugate base HC\(aq) is the acid, H3O* (ag) is its conjugate base HC(aq) is the acid, Cl" (ag) is its conjugate base
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.81QE: Write the chemical equation and the expression for the equilibrium constant, and calculate Kb for...
Related questions
Question
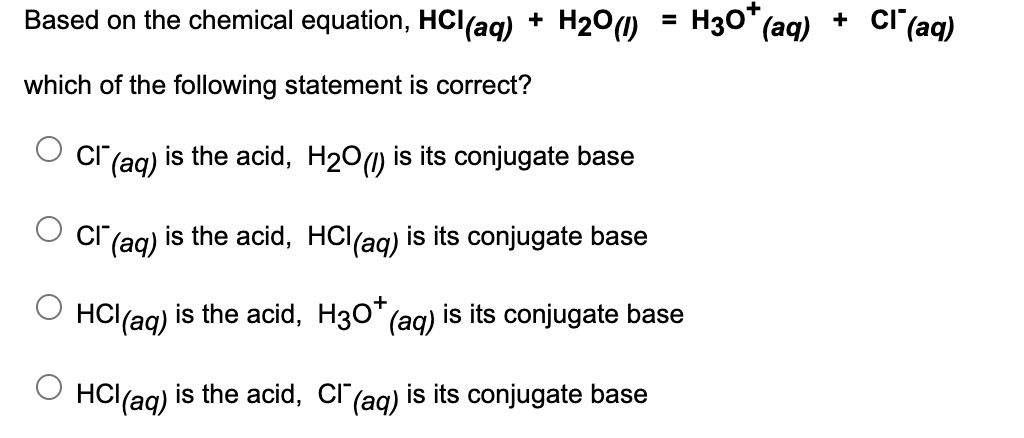
Transcribed Image Text:Based on the chemical equation, HCl(ag) + H200)
H30*
(aq)
cl (aq)
+
which of the following statement is correct?
|(aq)
is the acid, H20(1) is its conjugate base
|(aq)
is the acid, HCl(ag) is its conjugate base
HCl(ag) is the acid, H30* (ag) is its conjugate base
HCl(aq)
is the acid, Cl"(ag) is its conjugate base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning