Based on the thermodynamic properties provided for water, determine the energy change when the temperature of 1.35 kg of water decreased from 121 °C to 30.0 °C Value Property Units Melting point °C Boiling point 100.0 °C ΔΗus 6.01 kJ/mol AHvap kJ/mol 40.67 (s) 37.1 J/mol.C J/mol.C 75.3 Сp (s) 33.6 J/mol.C kJ
Based on the thermodynamic properties provided for water, determine the energy change when the temperature of 1.35 kg of water decreased from 121 °C to 30.0 °C Value Property Units Melting point °C Boiling point 100.0 °C ΔΗus 6.01 kJ/mol AHvap kJ/mol 40.67 (s) 37.1 J/mol.C J/mol.C 75.3 Сp (s) 33.6 J/mol.C kJ
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 102SCQ
Related questions
Question
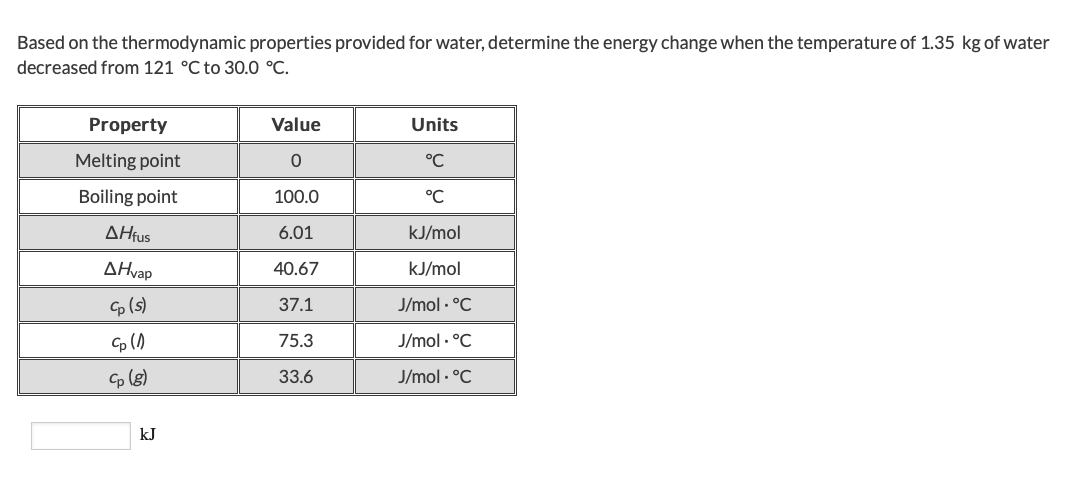
Transcribed Image Text:Based on the thermodynamic properties provided for water, determine the energy change when the temperature of 1.35 kg of water
decreased from 121 °C to 30.0 °C
Value
Property
Units
Melting point
°C
Boiling point
100.0
°C
ΔΗus
6.01
kJ/mol
AHvap
kJ/mol
40.67
(s)
37.1
J/mol.C
J/mol.C
75.3
Сp (s)
33.6
J/mol.C
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning