Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 138AE: One of the chemical controversies of the nineteenth century concerned the element beryllium (Be)....
Related questions
Question
Transcribed Image Text:M Che X
GA Hon X
A OW X
A Cha X
a http x OW X
A My x
E Clas X
E Clas X
enow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take
[Review Topics)
[References)
Use the References to access important values if needed for this question.
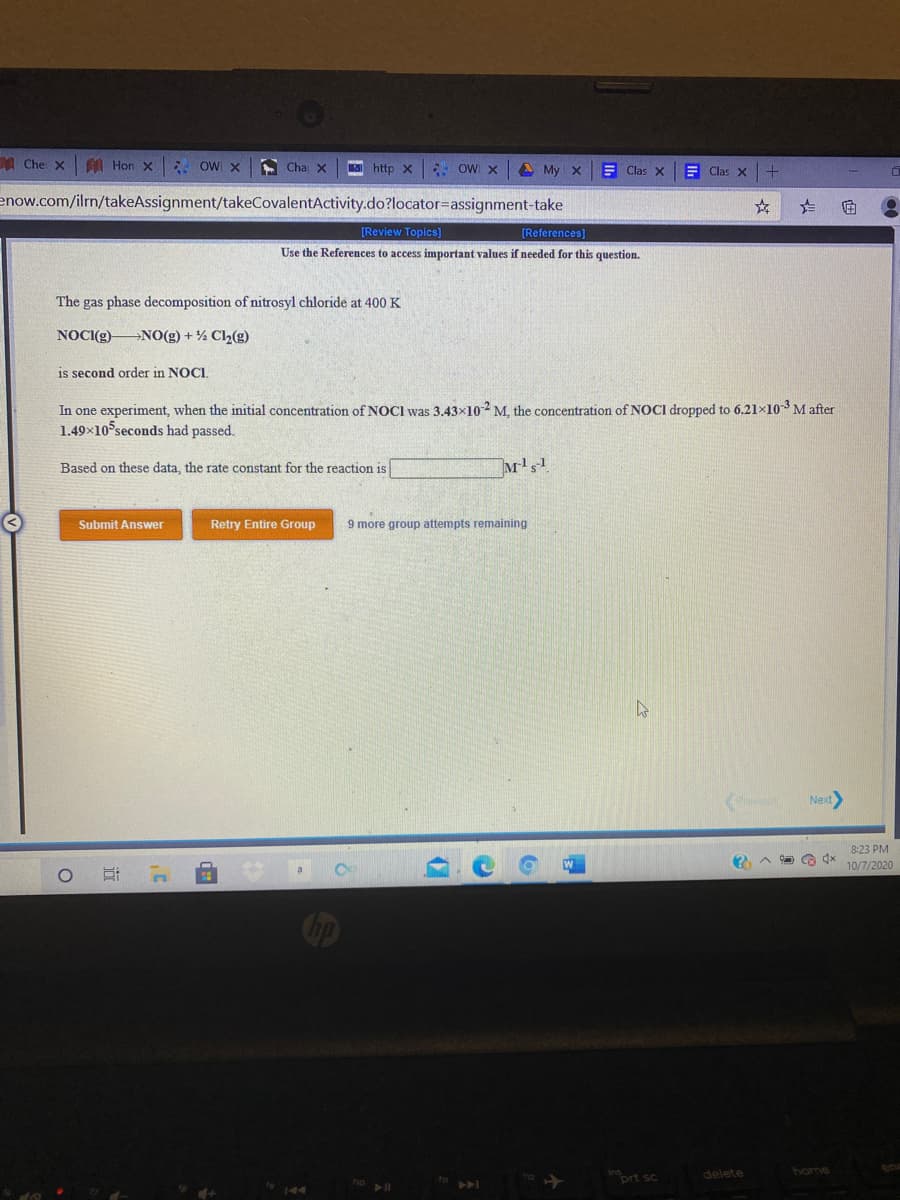
The gas phase decomposition of nitrosyl chloride at 400 K
NOCI(g)
NO(g) + Ch(g)
is second order in NOCI.
In one experiment, when the initial concentration of NOCI was 3.43×102 M, the concentration of NOCI dropped to 6.21×10³M after
1.49×10 seconds had passed.
Based on these data, the rate constant for the reaction is
Submit Answer
Retry Entire Group
9 more group attempts remaining
Next
8:23 PM
10/7/2020
home
enc
inrt sc
delete
144
近
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
