Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "submit". Predict if a reaction will occur when Hg(1) and hydrochloric acid (aq) are combined. If a reaction occurs, write a balanced equation for the reaction. + +
Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "submit". Predict if a reaction will occur when Hg(1) and hydrochloric acid (aq) are combined. If a reaction occurs, write a balanced equation for the reaction. + +
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.12QAP
Related questions
Question
23
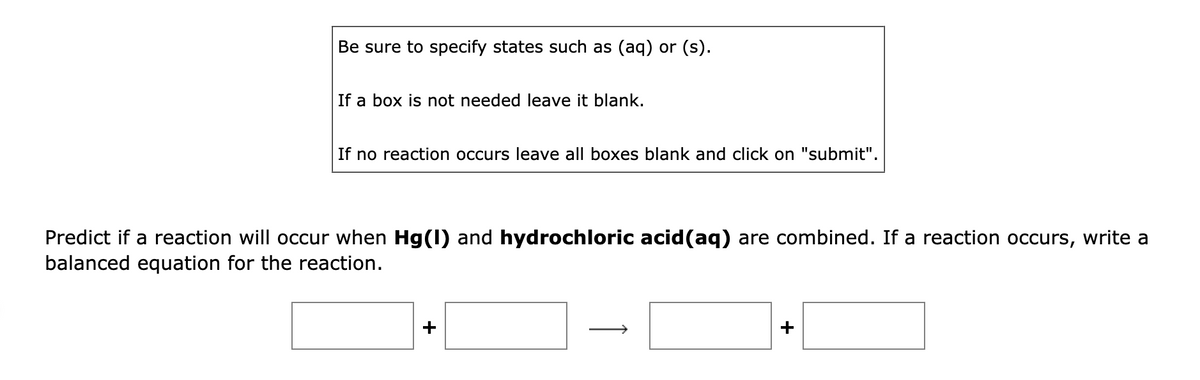
Transcribed Image Text:Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
If no reaction occurs leave all boxes blank and click on "submit".
Predict if a reaction will occur when Hg(1) and hydrochloric acid(aq) are combined. If a reaction occurs, write a
balanced equation for the reaction.
+
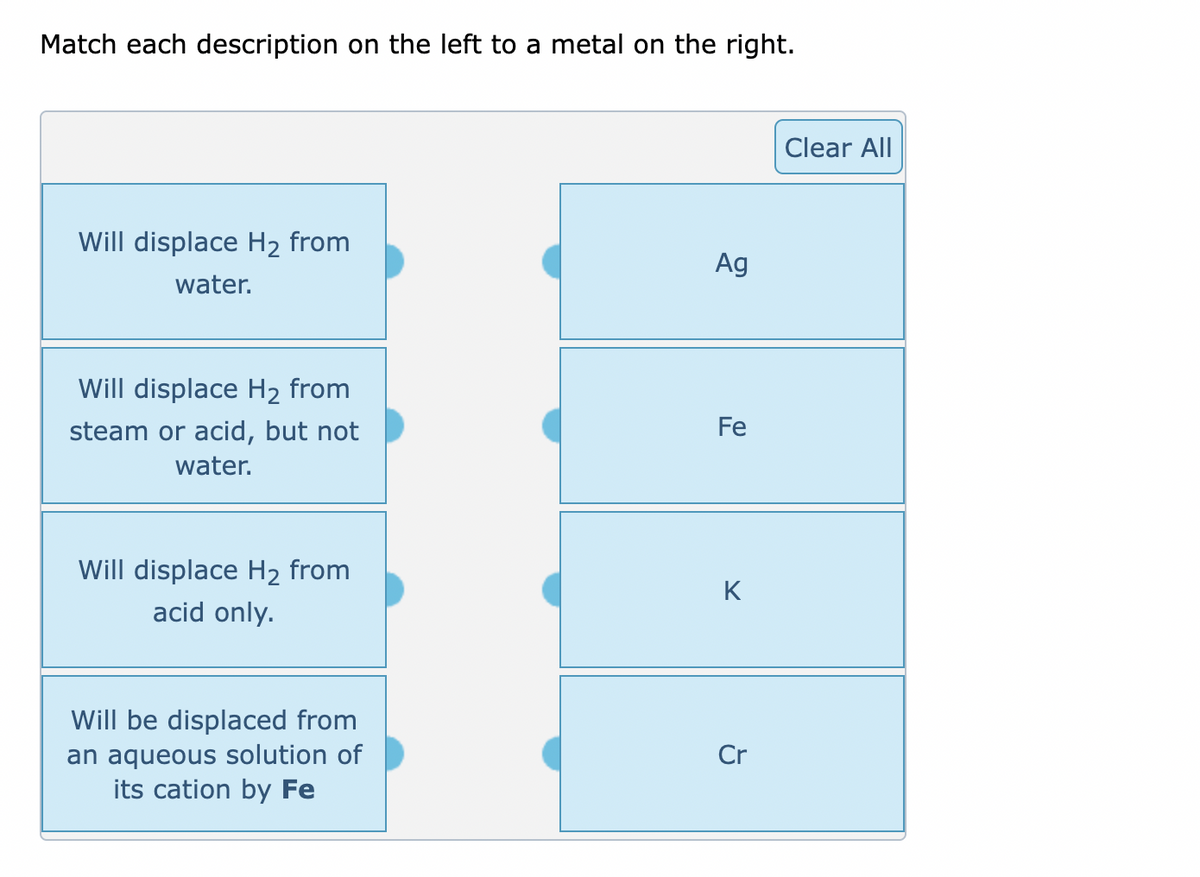
Transcribed Image Text:Match each description on the left to a metal on the right.
Will displace H₂ from
water.
Will displace H₂ from
steam or acid, but not
water.
Will displace H₂ from
acid only.
Will be displaced from
an aqueous solution of
its cation by Fe
Ag
Fe
K
Cr
Clear All
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning