Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron acceptor. ● NO₂ + 6e-> NH4+ (+0.34 volts) • O₂ +4e-> 2H₂O (+0.82 volts) Using the information given, calculate the AE for this reaction, balance the full reaction to determine the n, the number of electrons transferred when 30 moles of NH4* are oxidized. Finally, use the simplified Nernst Equation AG = -nFAE, where F = 96.5 kJ (mol e x V)-¹ to determine the Gibbs Free energy available to do work! • Report your answer in kJ rounded to two decimal places. o Include trailing zeros!! Always report two decimal places even if the answer is a whole number o e.g. 18.00 not 18 • Report only the numeric portion of your answer o e.g. 1.01, not 1.01 kj per mole. • Answers should ALWAYS be negative since this is a spontaneous reaction. Type your answer
Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron acceptor. ● NO₂ + 6e-> NH4+ (+0.34 volts) • O₂ +4e-> 2H₂O (+0.82 volts) Using the information given, calculate the AE for this reaction, balance the full reaction to determine the n, the number of electrons transferred when 30 moles of NH4* are oxidized. Finally, use the simplified Nernst Equation AG = -nFAE, where F = 96.5 kJ (mol e x V)-¹ to determine the Gibbs Free energy available to do work! • Report your answer in kJ rounded to two decimal places. o Include trailing zeros!! Always report two decimal places even if the answer is a whole number o e.g. 18.00 not 18 • Report only the numeric portion of your answer o e.g. 1.01, not 1.01 kj per mole. • Answers should ALWAYS be negative since this is a spontaneous reaction. Type your answer
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter19: Principles Of Chemical Reactivity: Electron Transfer Reactions
Section19.9: Corrosion: Redox Reactions In The Environment
Problem 2.4ACP: The overall reaction for the production of Cu(OH)2 from Cu in oxygenated water can be broken into...
Related questions
Question
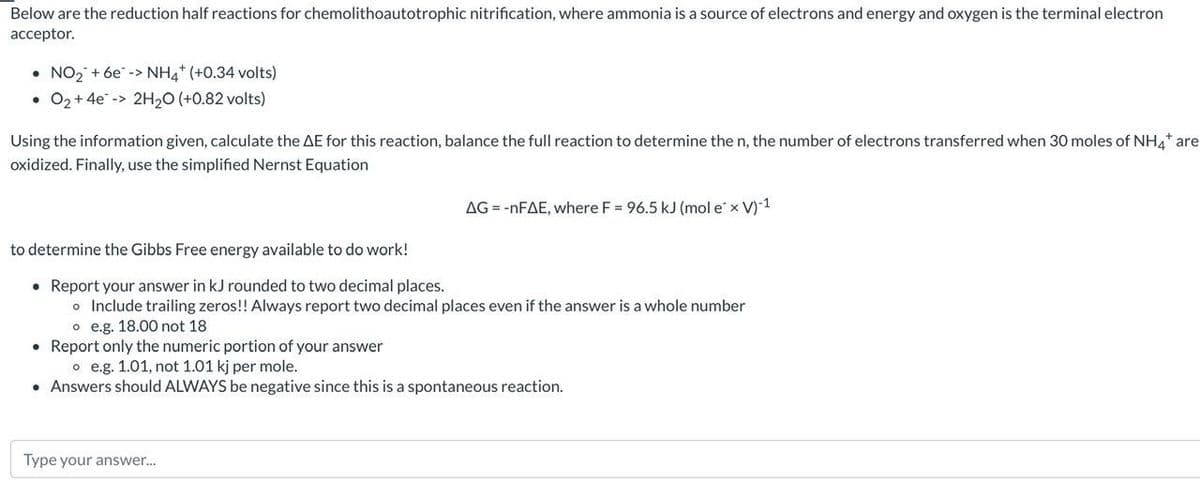
Transcribed Image Text:Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron
acceptor.
NO₂ + 6e-> NH4+ (+0.34 volts)
• O₂ + 4e -> 2H₂O (+0.82 volts)
●
Using the information given, calculate the AE for this reaction, balance the full reaction to determine the n, the number of electrons transferred when 30 moles of NH4* are
oxidized. Finally, use the simplified Nernst Equation
AG = -nFAE, where F = 96.5 kJ (mol e x V)-1
to determine the Gibbs Free energy available to do work!
• Report your answer in kJ rounded to two decimal places.
o Include trailing zeros!! Always report two decimal places even if the answer is a whole number
o e.g. 18.00 not 18
• Report only the numeric portion of your answer
o e.g. 1.01, not 1.01 kj per mole.
• Answers should ALWAYS be negative since this is a spontaneous reaction.
Type your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax