below each structure, write the hybridization state (sp, sp, or sp') =. ALL atoms and lone pair electrons are shown. H H *O: 'N' IN Br: H-Ö Ö-H s=c=o Br C: C: N: Hill C H C: H
below each structure, write the hybridization state (sp, sp, or sp') =. ALL atoms and lone pair electrons are shown. H H *O: 'N' IN Br: H-Ö Ö-H s=c=o Br C: C: N: Hill C H C: H
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.40QE: Use the VSEPR model to predict the bond angles around each central atom in the following Lewis...
Related questions
Question
100%
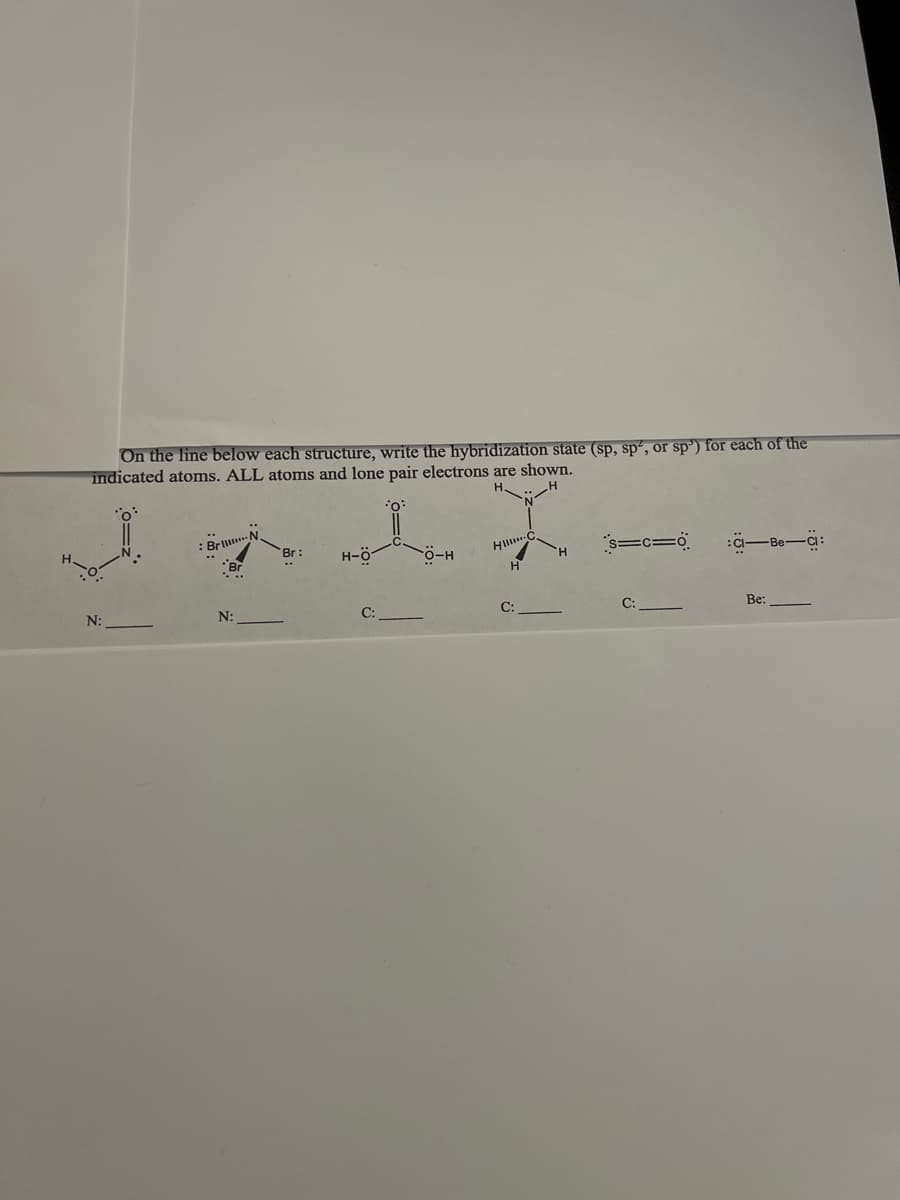
Transcribed Image Text:On the line below each structure, write the hybridization state (sp, sp², or sp') for each of the
indicated atoms. ALL atoms and lone pair electrons are shown.
.. H
0:
Bril
H
Br:
H-Ö
HI C
O-H
s=c=0
CI-Be-Ci:
H
N:
C:
Be:
N:
C:
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning