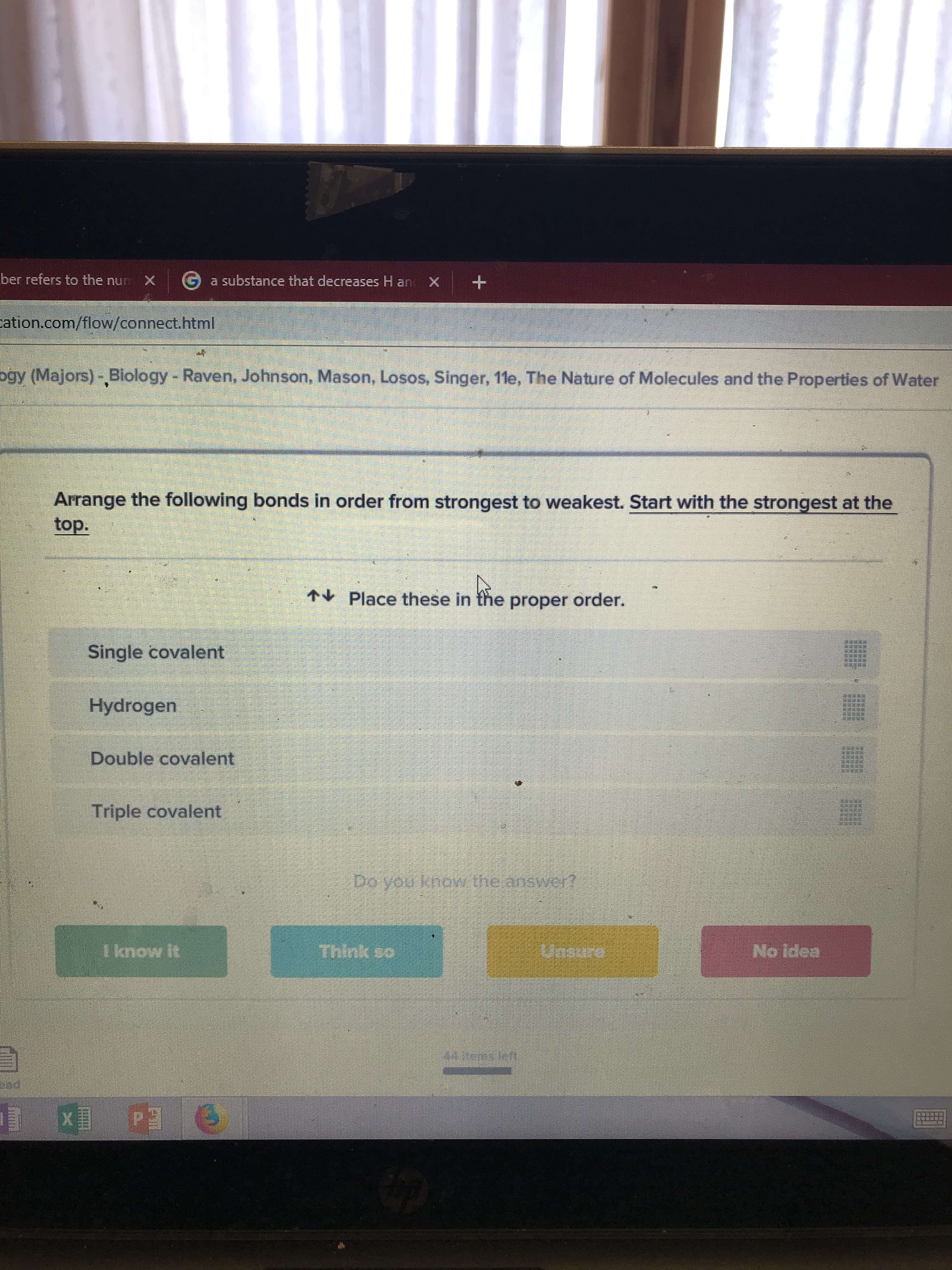
ber refers to the nur X ⓒ a substance that decreases H an | + ation.com/flow/connect.html ogy (Majors)-Biology -Raven, Johnson, Mason, Losos, Singer, 11e, The Nature of Molecules and the Properties of Water Arrange the following bonds in order from strongest to weakest. Start with the strongest at the top. 个 Place these in the proper order. Single covalent Hydrogen Double covalent Triple covalent Do you khow the answer? know it Think so No idea
ber refers to the nur X ⓒ a substance that decreases H an | + ation.com/flow/connect.html ogy (Majors)-Biology -Raven, Johnson, Mason, Losos, Singer, 11e, The Nature of Molecules and the Properties of Water Arrange the following bonds in order from strongest to weakest. Start with the strongest at the top. 个 Place these in the proper order. Single covalent Hydrogen Double covalent Triple covalent Do you khow the answer? know it Think so No idea
Human Biology (MindTap Course List)
11th Edition
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cecie Starr, Beverly McMillan
Chapter2: Chemistry Of Life
Section: Chapter Questions
Problem 10SQ: What kinds of bonds often control the shape (or tertiary form) of large molecules such as proteins?...
Related questions
Question
Transcribed Image Text:ber refers to the nur
X
ⓒ a substance that decreases H an
|
+
ation.com/flow/connect.html
ogy
(Majors)-Biology -Raven, Johnson, Mason, Losos, Singer, 11e, The Nature of Molecules and the Properties of Water
Arrange the following bonds in order from strongest to weakest. Start with the strongest at the
top.
个
Place these in the proper order.
Single covalent
Hydrogen
Double covalent
Triple covalent
Do you khow the answer?
know it
Think so
No idea
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Human Biology (MindTap Course List)
Biology
ISBN:
9781305112100
Author:
Cecie Starr, Beverly McMillan
Publisher:
Cengage Learning


Human Biology (MindTap Course List)
Biology
ISBN:
9781305112100
Author:
Cecie Starr, Beverly McMillan
Publisher:
Cengage Learning
