Boyle's Law Ľ states that for a certain gas in a container we have P. V = 345 where P represents the pressure of the gas (in mmHG) and V represents the volume of the gas (in liters). a. If the pressure of the gas is 220 mmHG, what is the volume of the gas? liters Preview b. Write a function f that determines the volume of the gas (in liters) in terms of the pressure of the gas in mmHG, P. f(P) = Preview c. Complete the following statement. (Hint: if f(P) increases without bound, enter "oo". If f(P) decreases without bound, enter "- o0".) As P → 0*, f(P) → Preview
Boyle's Law Ľ states that for a certain gas in a container we have P. V = 345 where P represents the pressure of the gas (in mmHG) and V represents the volume of the gas (in liters). a. If the pressure of the gas is 220 mmHG, what is the volume of the gas? liters Preview b. Write a function f that determines the volume of the gas (in liters) in terms of the pressure of the gas in mmHG, P. f(P) = Preview c. Complete the following statement. (Hint: if f(P) increases without bound, enter "oo". If f(P) decreases without bound, enter "- o0".) As P → 0*, f(P) → Preview
Big Ideas Math A Bridge To Success Algebra 1: Student Edition 2015
1st Edition
ISBN:9781680331141
Author:HOUGHTON MIFFLIN HARCOURT
Publisher:HOUGHTON MIFFLIN HARCOURT
Chapter10: Radical Functions And Equations
Section: Chapter Questions
Problem 15CT
Related questions
Question
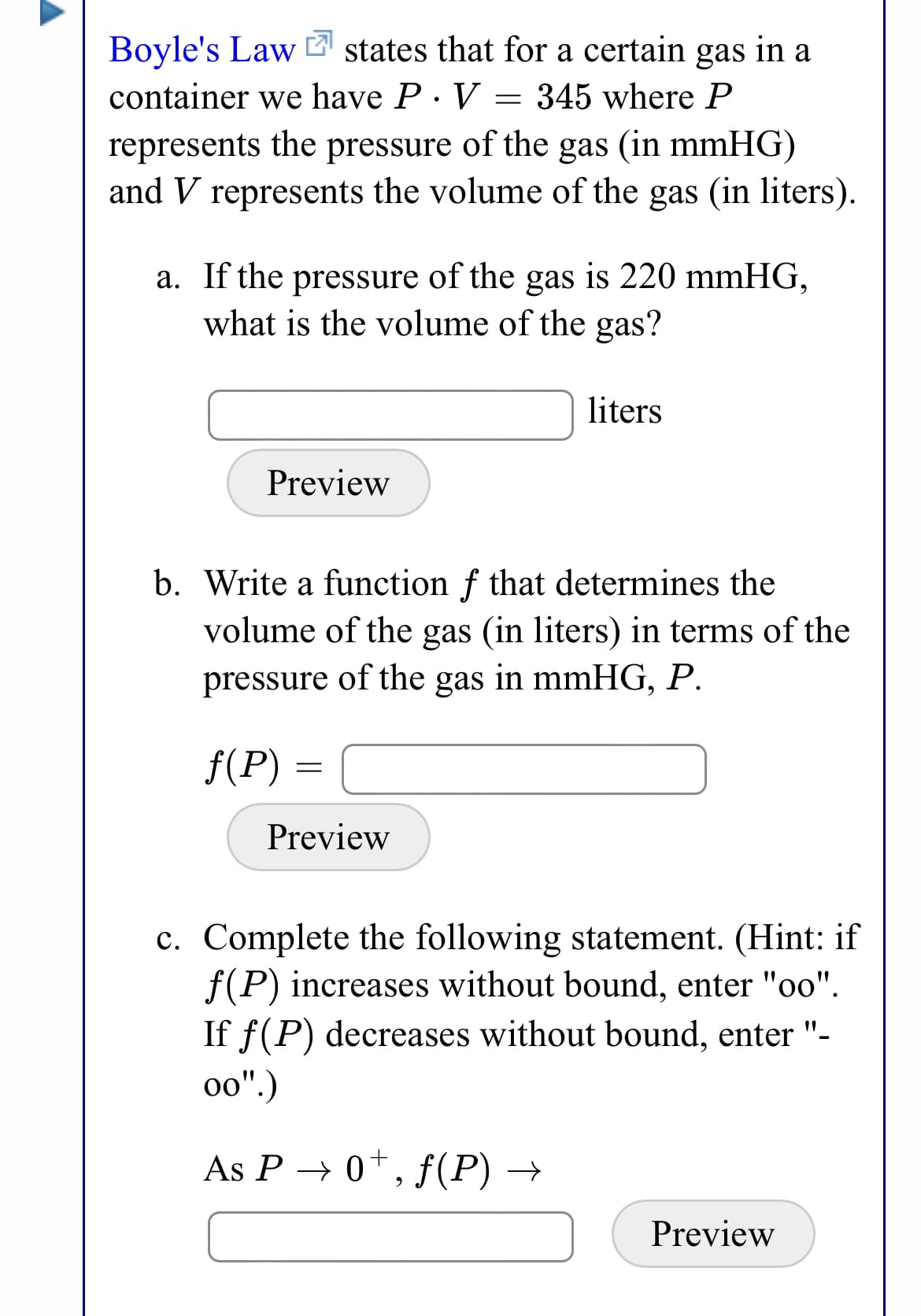
Transcribed Image Text:Boyle's Law states that for a certain gas in a
container we have P· V = 345 where P
represents the pressure of the gas (in mmHG)
and V represents the volume of the gas (in liters).
a. If the pressure of the gas is 220 mmHG,
what is the volume of the gas?
liters
Preview
b. Write a function f that determines the
volume of the gas (in liters) in terms of the
pressure of the gas in mmHG, P.
f(P) =
Preview
c. Complete the following statement. (Hint: if
f(P) increases without bound, enter "oo".
If f(P) decreases without bound, enter "-
o0".)
As P → 0*, f(P) →
Preview
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Big Ideas Math A Bridge To Success Algebra 1: Stu…
Algebra
ISBN:
9781680331141
Author:
HOUGHTON MIFFLIN HARCOURT
Publisher:
Houghton Mifflin Harcourt

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781337278461
Author:
Ron Larson
Publisher:
Cengage Learning


Big Ideas Math A Bridge To Success Algebra 1: Stu…
Algebra
ISBN:
9781680331141
Author:
HOUGHTON MIFFLIN HARCOURT
Publisher:
Houghton Mifflin Harcourt

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781337278461
Author:
Ron Larson
Publisher:
Cengage Learning


Glencoe Algebra 1, Student Edition, 9780079039897…
Algebra
ISBN:
9780079039897
Author:
Carter
Publisher:
McGraw Hill

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

College Algebra (MindTap Course List)
Algebra
ISBN:
9781305652231
Author:
R. David Gustafson, Jeff Hughes
Publisher:
Cengage Learning