Br2(g) 13. At 900 K, the following reaction 2 SO2 + O2 has Kp = 0.34: In an equilibrium mixture, the partial pressure of SO2 and 02 are 0.135 atm and 0.455 atm, respectively. What is the equilibrium partial pressure of SO3 in the mixture? A.0.0353 atm 2 SO3 B. 0.0535 atm 14. From the given Figure below, what conclusion you make? C. 0.1253 atm D.0.3553 atm noit 2 l u silnsu forward reaction equilibrium achieved T01 bob gear no Swene ilups ubor of obio Ja0ba backward reaction t hodqe e time bluor wod A. The forward and backward reactions are equal during the start of the chemical reaction. B. The forward and backward reactions are equal at the end of the chemical reaction. C. When the rates of forward and backward reactions reach the same point, chemical equilibrium is attained. D. When the rates of forward and backward reactions are in the highest and lowest points respectively, the 1'1 inod Reaction Rate
Br2(g) 13. At 900 K, the following reaction 2 SO2 + O2 has Kp = 0.34: In an equilibrium mixture, the partial pressure of SO2 and 02 are 0.135 atm and 0.455 atm, respectively. What is the equilibrium partial pressure of SO3 in the mixture? A.0.0353 atm 2 SO3 B. 0.0535 atm 14. From the given Figure below, what conclusion you make? C. 0.1253 atm D.0.3553 atm noit 2 l u silnsu forward reaction equilibrium achieved T01 bob gear no Swene ilups ubor of obio Ja0ba backward reaction t hodqe e time bluor wod A. The forward and backward reactions are equal during the start of the chemical reaction. B. The forward and backward reactions are equal at the end of the chemical reaction. C. When the rates of forward and backward reactions reach the same point, chemical equilibrium is attained. D. When the rates of forward and backward reactions are in the highest and lowest points respectively, the 1'1 inod Reaction Rate
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 113QRT
Related questions
Question
Answer number 13-14
Transcribed Image Text:2(8)
Br2(g)
13. At 900 K, the following reaction 2 SO2 + O2 =
has Kp 0.34: In an equilibrium mixture, the partial pressure of SO2 and 02 are 0.135 atm and 0.455 atm,
respectively. What is the equilibrium partial pressure of SO3 in the mixture?
A. 0.0353 atm
2 SO3
B. 0.0535 atm
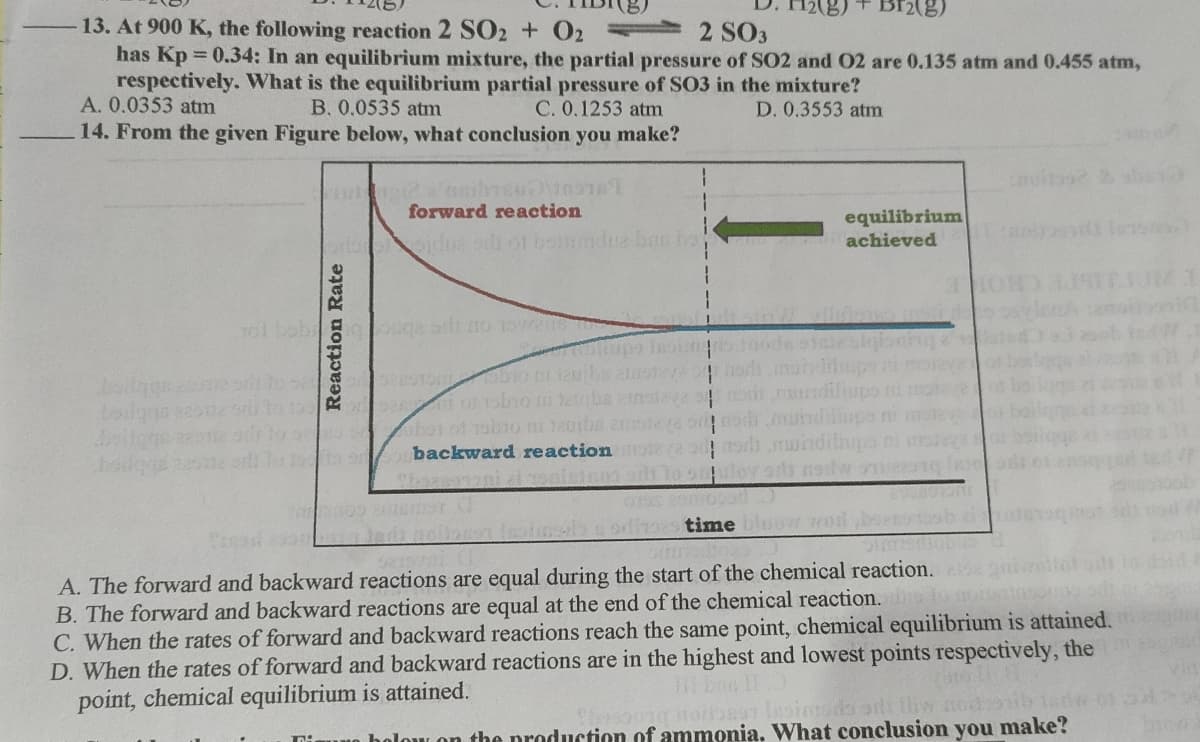
14. From the given Figure below, what conclusion you make?
C. 0.1253 atm
D. 0.3553 atm
1.
forward reaction
equilibrium
achieved
brloelfoidue of boinmdua ban bo
101 bobi
esigionhg
30. 0 bor ol obio n Ja0ba
backward reaction
wndiliups ti
undilinps ri
whdilapa ni
time bluov wod bs
A. The forward and backward reactions are equal during the start of the chemical reaction.
B. The forward and backward reactions are equal at the end of the chemical reaction.
C. When the rates of forward and backward reactions reach the same point, chemical equilibrium is attained.
D. When the rates of forward and backward reactions are in the highest and lowest points respectively, the
point, chemical equilibrium is attained.
oibesr lesimsdo si tliw nooib lar
holow on the production of ammonia. What conclusion you make?
Reaction Rate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning