Bromine reacts with alkenes in methanol according to the equation: - When this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield). Which of the following is the structure more reasonable for this compound?. Explain your reasoning through a corresponding mechanism.
Bromine reacts with alkenes in methanol according to the equation: - When this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield). Which of the following is the structure more reasonable for this compound?. Explain your reasoning through a corresponding mechanism.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter19: Transitition Metals, Coordination Chemistry And Metallurgy
Section: Chapter Questions
Problem 19.41QE
Related questions
Question
Bromine reacts with
- When this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield). Which of the following is the structure more reasonable for this compound?. Explain your reasoning through a
corresponding mechanism.
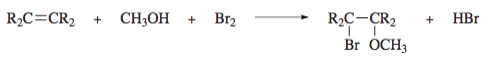
Transcribed Image Text:R2C=CR2
CH;OH
Br2
R2C-CR2
HBr
+
+
Br OCH3
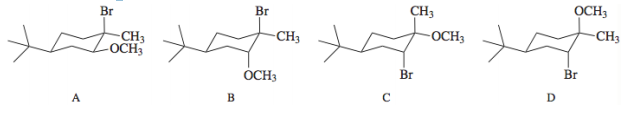
Transcribed Image Text:Br
Br
CH3
OCH3
-CH3
-CH3
-OCH3
-OCH3
CH3
ÓCH3
Br
Br
А
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning