By using the Poiseuille equation for gas viscosity determination, the obtained CO2 gas viscosity at 20 and 60 ° C are 147 and 166 µP, respectively, with the help of the kinetic theory of gases' equation, (RTM) (NT ) what would be the estimated CO2 molecular diameter (o) in pm? (Atomic masses: C = 12 and O =16 amu, and R = 8.314 J K-1 mol-1). = 2.96x10-25 (RTM)'? O A. 940 pm О В. 398 pm о с. 327 pm OD.870 pm O E. 233 pm
By using the Poiseuille equation for gas viscosity determination, the obtained CO2 gas viscosity at 20 and 60 ° C are 147 and 166 µP, respectively, with the help of the kinetic theory of gases' equation, (RTM) (NT ) what would be the estimated CO2 molecular diameter (o) in pm? (Atomic masses: C = 12 and O =16 amu, and R = 8.314 J K-1 mol-1). = 2.96x10-25 (RTM)'? O A. 940 pm О В. 398 pm о с. 327 pm OD.870 pm O E. 233 pm
Related questions
Question
1.
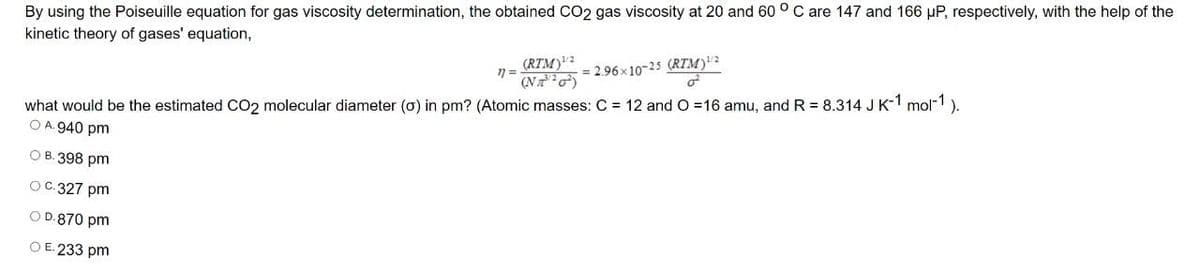
Transcribed Image Text:By using the Poiseuille equation for gas viscosity determination, the obtained CO2 gas viscosity at 20 and 60 ° C are 147 and 166 µP, respectively, with the help of the
kinetic theory of gases' equation,
(RTM)?
(NT )
= 2.96x10-25 (RTM)"2
what would be the estimated CO2 molecular diameter (o) in pm? (Atomic masses: C = 12 and O 16 amu, and R = 8.314 J K-1 mol-1).
O A. 940 pm
О В. 398 pm
ОС. 327 pm
O D.870 pm
O E. 233 pm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
