C2 HCl, 241.93 g/mol Express your answer as a chemical formula. ΑΣφ ? OA chemical reaction does not occur for this question. Submit Request Answer Part C C5 H10 NS2, 296.54 g/mol Express your answer as a chemical formula. ΑΣφ ? A chemical reaction does not occur for this question.
C2 HCl, 241.93 g/mol Express your answer as a chemical formula. ΑΣφ ? OA chemical reaction does not occur for this question. Submit Request Answer Part C C5 H10 NS2, 296.54 g/mol Express your answer as a chemical formula. ΑΣφ ? A chemical reaction does not occur for this question.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter3: Stoichiometry
Section: Chapter Questions
Problem 13ALQ: What is true about the chemical properties of the product? a. The properties are more like chemical...
Related questions
Question
Find the molecular formula or each given compound. Please help with both. Thank you.
Transcribed Image Text:8:29 G G G o d
N all i
e MasteringChemistry: CH 3 Homew...
session.masteringchemistry.com
<CH 3 Homework
Exercise 3.95
22 of 25
>
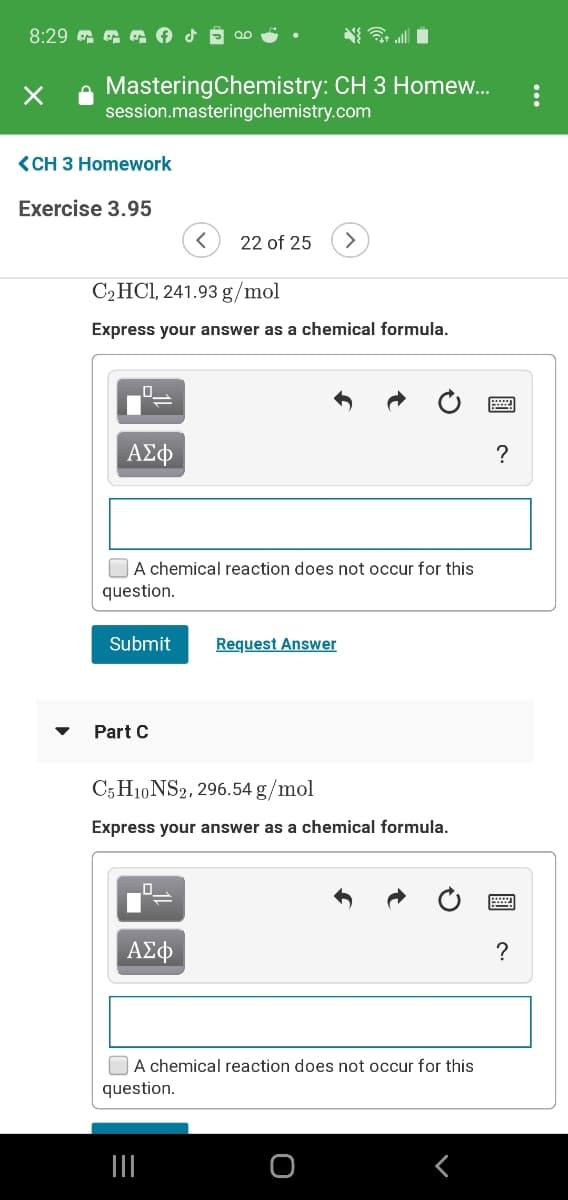
C2HCI, 241.93 g/mol
Express your answer as a chemical formula.
ΑΣφ
?
OA chemical reaction does not occur for this
question.
Submit
Request Answer
Part C
C5 H10 NS2, 296.54 g/mol
Express your answer as a chemical formula.
?
OA chemical reaction does not occur for this
question.
...
Expert Solution
Step 1
For 1st compound,
Empirical Formula = C2HCl
Empirical formula Mass(Emass) = (2 x 12) + 1 +35.5 = 60.5 gms
Given Molecular Formula Mass(Mmass) = 241.93 gms/mol
The molecular formula can be represented by:
Empirical formula mass and molecular formula are related by the below given expression.
Now, putting the known values, we get
Putting the Value of N in the above mentioned representation, we get the molecular formula as .
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning