Calcium sulfate, CaSO4, has a Ksp value of 7.10 × 10¬³. What happens when calcium and sulfate solutions are mixed to give 2.00 × 10–³ M Ca²+ and 3.00 x 10-² MSO4²-? A precipitate forms because Q > Ksp. A precipitate forms because Q < Ksp. No precipitate forms because Q > Ksp. No precipitate forms because Q < Ksp.
Calcium sulfate, CaSO4, has a Ksp value of 7.10 × 10¬³. What happens when calcium and sulfate solutions are mixed to give 2.00 × 10–³ M Ca²+ and 3.00 x 10-² MSO4²-? A precipitate forms because Q > Ksp. A precipitate forms because Q < Ksp. No precipitate forms because Q > Ksp. No precipitate forms because Q < Ksp.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.97QE: According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as...
Related questions
Question
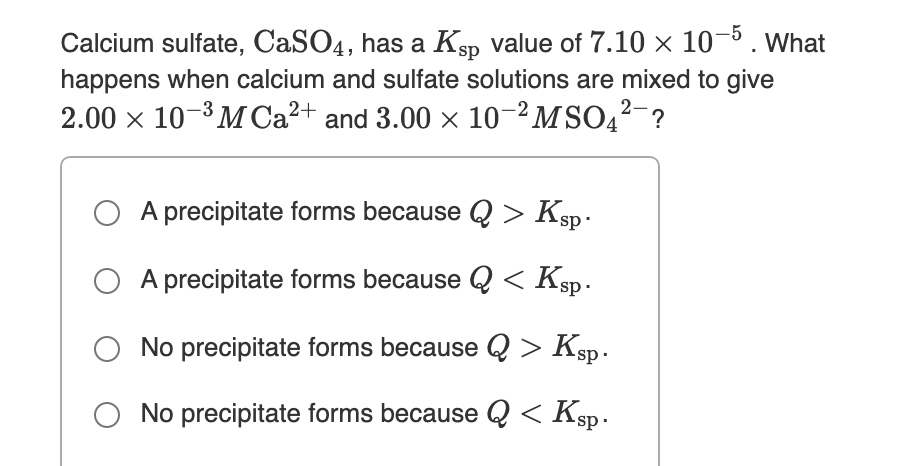
Transcribed Image Text:Calcium sulfate, CaSO4, has a Ksp value of 7.10 × 10-5. What
happens when calcium and sulfate solutions are mixed to give
2.00 x 10-3 MCA²+ and 3.00 x 10–²MSO4?-?
A precipitate forms because Q > Ksp .
A precipitate forms because Q < Ksp.
No precipitate forms because Q > Ksp.
O No precipitate forms because Q < Ksp.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax