Calculate AS when 3.2 moles of n-hexane goes from 298 K and 1 atm to 398 K and 2 atm. The table below provides useful data, all at 298 K and 1 atm (reference: Cerdeiriña, Claudio A., et al. "Isobaric thermal expansivity and thermophysical characterization of liquids and liquid mixtures." Physical Chemistry Chemical Physics 3.23 (2001): 5230-5236). You may neglect the pressure and temperature dependence of all quantities. Compound Molar Volume (cm³/mol) Isothermal Compressibility (1/atm) Thermal Expansity (1/K) Cp,m (J/molK) n-hexane 133.09 1.69 x 10-4 1.38 x 10-3 195.66 n-heptane 147.45 1.47 x 10-4 1.246 x 10-3 224.64 n-dodecane 228.55 1.00 x 10-4 9.69 x 10-4 376.22
Calculate AS when 3.2 moles of n-hexane goes from 298 K and 1 atm to 398 K and 2 atm. The table below provides useful data, all at 298 K and 1 atm (reference: Cerdeiriña, Claudio A., et al. "Isobaric thermal expansivity and thermophysical characterization of liquids and liquid mixtures." Physical Chemistry Chemical Physics 3.23 (2001): 5230-5236). You may neglect the pressure and temperature dependence of all quantities. Compound Molar Volume (cm³/mol) Isothermal Compressibility (1/atm) Thermal Expansity (1/K) Cp,m (J/molK) n-hexane 133.09 1.69 x 10-4 1.38 x 10-3 195.66 n-heptane 147.45 1.47 x 10-4 1.246 x 10-3 224.64 n-dodecane 228.55 1.00 x 10-4 9.69 x 10-4 376.22
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 81AP: One process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of...
Related questions
Question
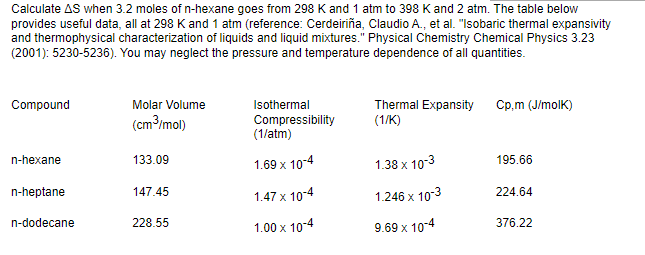
Transcribed Image Text:Calculate AS when 3.2 moles of n-hexane goes from 298 K and 1 atm to 398 K and 2 atm. The table below
provides useful data, all at 298 K and 1 atm (reference: Cerdeiriña, Claudio A., et al. "Isobaric thermal expansivity
and thermophysical characterization of liquids and liquid mixtures." Physical Chemistry Chemical Physics 3.23
(2001): 5230-5236). You may neglect the pressure and temperature dependence of all quantities.
Compound
Molar Volume
(cm³/mol)
Isothermal
Compressibility
(1/atm)
Thermal Expansity
(1/K)
Cp,m (J/molK)
n-hexane
133.09
1.69 x 10-4
1.38 x 10-3
195.66
n-heptane
147.45
1.47 x 10-4
1.246 x 10-3
224.64
n-dodecane
228.55
1.00 x 10-4
9.69 x 10-4
376.22
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning