Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 121AP: Calcium carbonate, CaCO3, can be obtained in a very pure state. Standard solutions of calcium ion...
Related questions
Question
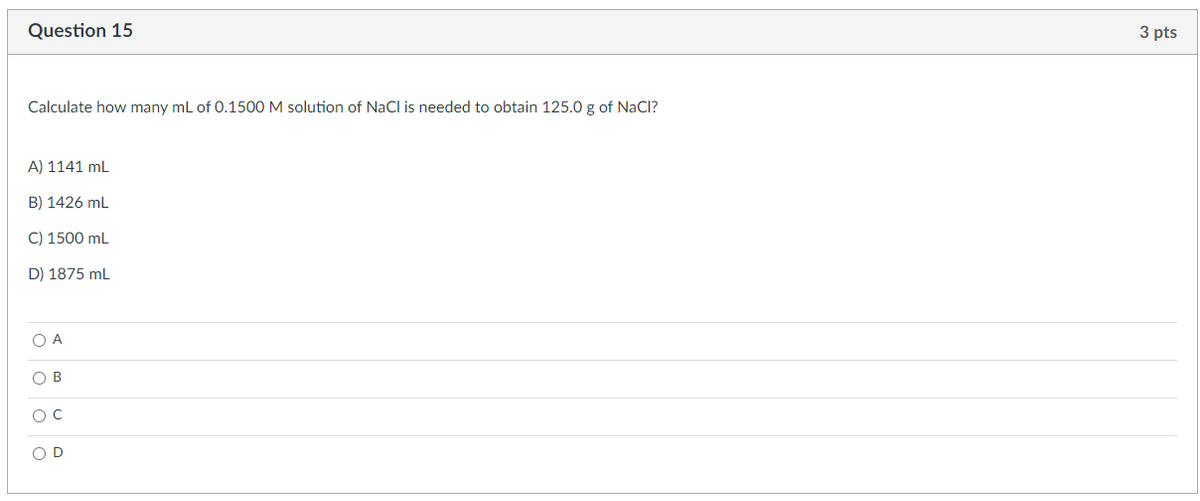
Transcribed Image Text:Question 15
3 pts
Calculate how many ml of 0.1500 M solution of NaCl is needed to obtain 125.0 g of NaCI?
A) 1141 mL
B) 1426 mL
C) 1500 mL
D) 1875 mL
O A
O B
OD
Expert Solution
Step 1
From the definition of molarity we have 0.1500 M = 0.1500 moles /1000 mL
Now we will convert moles into grams by multiplying with molar mass of NaCl.
Mass = moles * molar mass of NaCl
= 0.1500 moles* 58.44 g/moles
= 8.766 g
So, 0.1500 M = 8.766 g/ 1000 mL.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax