Calculate the AH, AS, and AG at 298 K for the following reaction: (Use the values found in Thermodynamic Properties to calculate your answers for questions a and b.) Al2O3(s) + 3 C(graphite) + 3 Cl₂(g) →2 AlCl3(s) + 3 CO(g) a) AH° 40-64 ✓ kJ/mol b) AS 40 76.9 c) AGO 49-89 ✓ J/K-mol ✔ kJ/mol (Determine from the temperature, AH and AS) (g) Estimate the value of K at 600 K. 5.59e7 X (Please answer to 3 significant figures.)
Calculate the AH, AS, and AG at 298 K for the following reaction: (Use the values found in Thermodynamic Properties to calculate your answers for questions a and b.) Al2O3(s) + 3 C(graphite) + 3 Cl₂(g) →2 AlCl3(s) + 3 CO(g) a) AH° 40-64 ✓ kJ/mol b) AS 40 76.9 c) AGO 49-89 ✓ J/K-mol ✔ kJ/mol (Determine from the temperature, AH and AS) (g) Estimate the value of K at 600 K. 5.59e7 X (Please answer to 3 significant figures.)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.97PAE
Related questions
Question
100%
Just part g that is incorrect
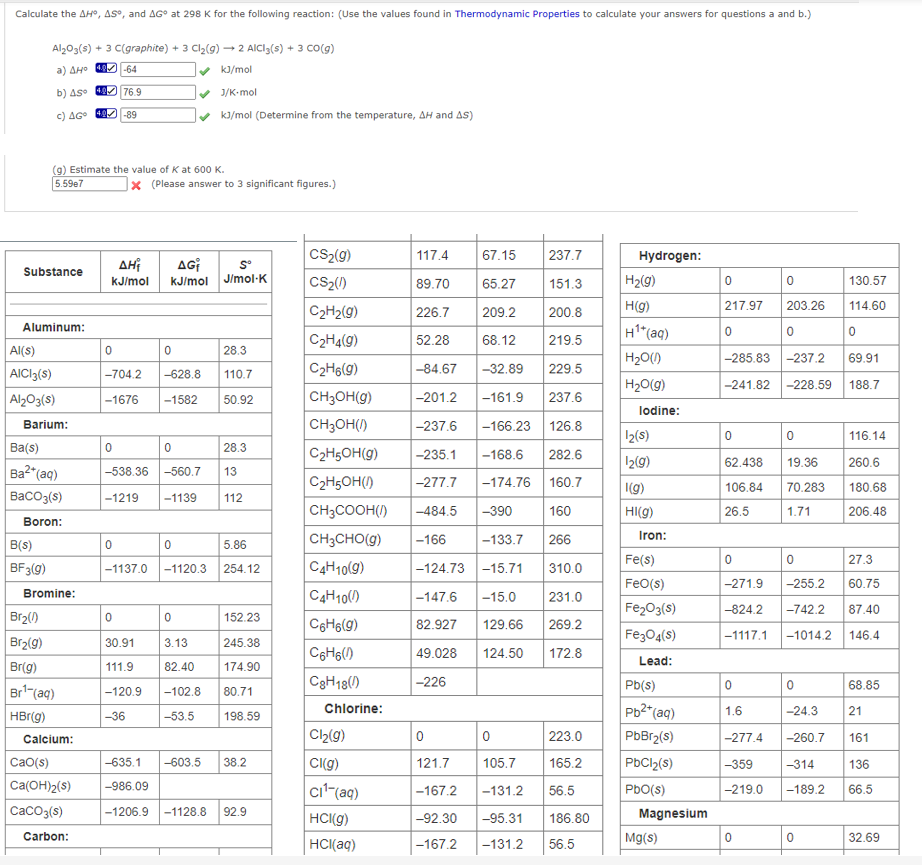
Transcribed Image Text:Calculate the AH, AS, and AG at 298 K for the following reaction: (Use the values found in Thermodynamic Properties to calculate your answers for questions a and b.)
Al2O3(s) + 3 C(graphite) + 3 Cl₂(g) →2 AlCl3(s) + 3 CO(g)
a) AH° 40-64
✓ kJ/mol
b) AS 40 76.9
c) AGO 49-89
Substance
(g) Estimate the value of K at 600 K.
5.59e7
Aluminum:
Al(s)
AICI 3(S)
Al₂O3(S)
Barium:
B(s)
BF 3(9)
Ba(s)
Ba2 (aq)
BaCO3(s)
Boron:
Bromine:
Carbon:
AH
AGI
kJ/mol kJ/mol
0
X (Please answer to 3 significant figures.)
0
28.3
-704.2 -628.8
110.7
-1676 -1582 50.92
0
28.3
-538.36 -560.7
13
-1219 -1139 112
-1137.0
Br₂()
Br₂(g)
Br(g)
Br¹-(aq)
HBr(g)
Calcium:
| CaO(s)
Ca(OH)₂(S) -986.09
CaCO3(s)
0
0
✓ J/K-mol
✔ kJ/mol (Determine from the temperature, AH and AS)
0
Sº
J/mol-K
0
5.86
-1120.3 254.12
0
152.23
245.38
174.90
30.91
3.13
111.9 82.40
-120.9 -102.8 80.71
-36
-53.5 198.59
-635.1 -603.5 38.2
-1206.9 -1128.8 92.9
CS₂(g)
CS₂(!)
C₂H₂(g)
C₂H4(9)
C₂H6(9)
CH3OH(g)
CH₂OH(1)
C₂H5OH(g)
C₂H5OH (1)
CH3COOH()
CH3CHO(g)
C4H10(9)
C4H10()
С6H₁(9)
C6H6(1)
CgH 18(1)
Chlorine:
Cl₂(g)
CI(g)
C₁¹-(aq)
HCI(g)
HCl(aq)
117.4 67.15
237.7
89.70 65.27
151.3
226.7 209.2 200.8
52.28 68.12 219.5
-84.67
229.5
-32.89
-201.2 -161.9 237.6
-237.6 -166.23 126.8
-235.1 -168.6 282.6
-277.7
-174.76
160.7
-484.5
-390
160
-166
-133.7
266
-124.73 -15.71 310.0
-147.6 -15.0
231.0
82.927 129.66 269.2
49.028 124.50 172.8
-226
0
223.0
121.7
105.7
165.2
-167.2 -131.2 56.5
-92.30 -95.31
186.80
-167.2 -131.2
56.5
0
Hydrogen:
H₂(g)
H(g)
H¹+ (aq)
H₂O(1)
H₂O(g)
lodine:
12(s)
¹2(9)
I(g)
HI(g)
Iron:
Fe(s)
FeO(s)
Fe₂O3(s)
Fe3O4(S)
Lead:
Pb(s)
Pb²+ (aq)
PbBr₂(s)
PbCl₂(S)
PbO(s)
Magnesium
Mg(s)
0
217.97
0
-285.83 -237.2
-241.82 -228.59
0
62.438
106.84
26.5
0
0
203.26
0
0
0
19.36
70.283
1.71
0
-271.9 -255.2
-824.2 -742.2
-1117.1 -1014.2
0
0
1.6
-24.3
-277.4 -260.7
-359 -314
-219.0 -189.2
0
130.57
114.60
0
69.91
188.7
116.14
260.6
180.68
206.48
27.3
60.75
87.40
146.4
68.85
21
161
136
66.5
32.69
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning