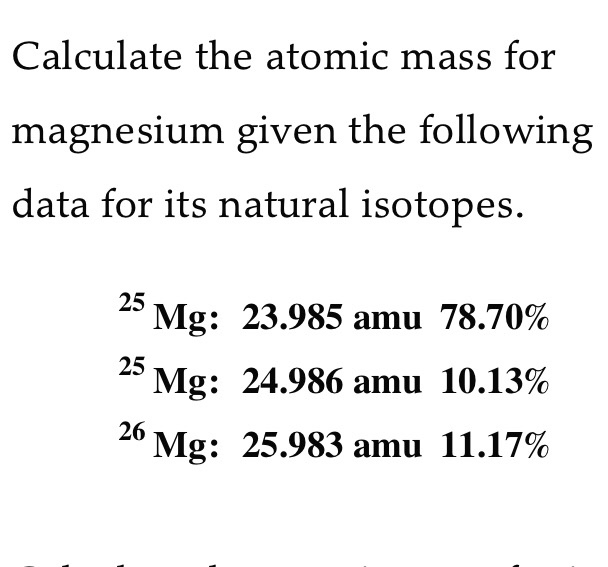
Calculate the atomic mass for magnesium given the following data for its natural isotopes. 25 Mg: 23.985 amu 78.70% 25 Mg: 24.986 amu 10.13% 26 Mg: 25.983 amu 11.17%
Q: How are the components of the acceleration of a projectile related to the mass of an object? How ar...
A: In the case of projectile motion, the horizontal acceleration is zero. and hence it is independen...
Q: If steel has ten times the density of water, how many times more dense is the hydrogen plasma at the...
A:
Q: Suppose that you have a 2D quantum system where X and Px are the x- component position and momentum ...
A: The commutator for Py and y Py,yψ=Pyy-yPyψ=Pyyψ-yPyψ=-iℏ∂∂y(yψ)-y(-iℏ∂∂y(ψ))=-iℏψ-iℏy∂ψ∂y+iℏy∂ψ∂y=-i...
Q: Draw a labelled free body diagram to a book is at rest on tabletop.
A:
Q: (i) The utilized reflecting plane of a lithium fluoride analyzing crystal has a interplanardistance ...
A: Hello. Since you have posted multiple questions and not specified which question needs to be solved,...
Q: The radioactive isotope 12 7N undergoes b+ decay. The energy released in the decay of one nucleus is...
A:
Q: A person ingests an amount of a radioactive source that has a very long lifetime and activity 0.52 µ...
A: absorbed dose rate is the amount of radiation energy absorbed by the tissue. Therefore absorbed dos...
Q: Suppose that you have the Lagrangian L = (;2 + 0ʻr²) + 420 for a 2D 20 system in plane polar coordin...
A: Conjugate momenta Pq corresponding to conjugate variable q is given by Where L = Lagrangian of the ...
Q: Write balanced nuclear equations for:1. the loss of a positron by Y-852. the alpha emission resultin...
A: The typical nuclear reactions corresponding to alpha decay and beta decay of a nucleus (X) may be ex...
Q: number of forces in plane: centripetal force: Are all forces balanced? How much force is acting on t...
A: Number of forces in plane = 2 (centripetal and normal force) yes the forces are balanced.
Q: Please provide answer urgently along with a detailed explanation
A: Frequency of the string f=12LTμ=12LTρVL=12LTρπr2LL=12LrTρπ=1LDTρπ 2r=D
Q: The capacitor in the figure below is initially uncharged. The switch S is closed at t = 0. (a) Expla...
A: Hello. Since your question has multiple sub-parts, we will solve first three sub-parts for you. If y...
Q: A 100 kg block of ice slides down an incline that is 5m long and 3m tall. Someone pushes up on the b...
A:
Q: Fission reactions occur only for nuclei with large nucleon numbers, while exoergic fusion reactions ...
A: The ratio of the neutron and proton for stable nuclides or light nuclides is: NZ=1 Here, N represent...
Q: calculate the weight of the hanging mass fg2
A: There are ten washers, each washer weighs 10 g. Therefore the total hanging mass is 10g ×10=100 g. T...
Q: A box is sliding with a constant speed of 4.00 m>s inthe +x-direction on a horizontal, frictionle...
A: Case-1:- When the box is sliding at coordinates, 0≤x≤2.00 m Three forces act on the box:- Normal for...
Q: A 12-pack of Omni-Cola (mass 4.30 kg) is initially at rest on a horizontal floor. It is then pushed ...
A: Part (a) Given: The mass of pack is m=4.30 kg. The distance covered by pack is d=1.20 m. The horizo...
Q: Light enters a solid pipe made of plastic having an index of refraction of 1.60. The light travels p...
A: (a) The expression for the critical angle to occur total internal reflection is as follows: θc=sin-1...
Q: You are standing on a large sheet of frictionless ice and holdinga large rock. In order to get off t...
A: The velocity of rock relative to earth vr = 12 m/s. The angle of velocity to the horizontal θ = 35o....
Q: An electromagnetic wave with frequency 65.0 Hz travels in an insulating magnetic material that has d...
A: (a)Speed of propagation of the electromagnetic wave in a material medium,v=cμ0ε0 ...
Q: The signal from the oscillating electrode is fed into an amplifier, which reports the measured volta...
A: The peak voltage is, vp=vrms2vp=1.5 nV2vp=2.1 nV
Q: What’s the center of mass for each situation?
A:
Q: I need helping with this! Thanks in advance! I think the answer is C but i just wanted to make sure!...
A: The highest equivalent capacitance is the results of two capacitors are in parallel with effective c...
Q: Make a sketch of the trajectory of a ball after it has been thrown. Draw the ball in at least five d...
A: (1) Introduction: A projectile is an object upon which the only force acting is gravity. There are a...
Q: What is the speed of a particle whose kinetic energy is equal to (a) its rest energy and (b) five ti...
A: The expression for the relativistic kinetic energy is given by, γKE=moc2+KEmoc21-v2c2=moc2+KE Here, ...
Q: A bull shark has a reduced weight (or “submerged weight” = W - B) of 4Nin seawater and 12N in freshw...
A: When a body flows in a fluid, the fluid exerts a force (aerodynamic force) on the surface of the bod...
Q: A particle P of mass m lies on a smooth horizontal table attached to a long string and inextensible ...
A: Let m denote the mass of the particle at point P, km denote the mass of the particle at point Q, a d...
Q: Select the direction of each external force acting on the highlighted object. Only include forces in...
A: weight will act downward normal force will act towards right (perpendicular to inner side of loop) T...
Q: Find the x and y coordinates of the centroid of the shape shown below via integration.
A: Solution: The x and y coordinates of centroid of the given shape is solved below: From the abov...
Q: A 60kg skateboarder (including the skateboard) wants to figure out how fast he needs to be traveling...
A: Mass of the skateboarder (including skateboard) m = 60 kg The diameter of the path d = 14 m
Q: Prove the following switching relationships: (а) [А, В-1] 3 - (b) [A, [B, C]] + [B,[C,A]] + [C,[A, B...
A: Hello. Since your question has multiple sub-parts, we will solve the first three sub-parts for you. ...
Q: Find the radii of the 238U and 4He nuclei and then determine the ratio of those radii.
A: The radii of the elements are, RU=RoAU13RU=1.2 fm23813RU=7.44 fmRHe=RoAHe13RHe=1.2 fm413RHe=1.90 fm
Q: A cow is leaving the barn as you try harder and harder to push her back in. In coordinates with the ...
A: Work done by the variable force on the system is given by Where x1 = Initial position, x2 = Final p...
Q: A cylinder measuring 7.8 cm wide and 9.4 cm high is filled with gas and the piston pushed down with ...
A: We know that Pressure =Force/Area The pressure in the gas is measured as =192kPa The piston is circu...
Q: Question in the attachment below
A: Given: The number of turns in the coil are 32. The radius of the coil is 7.81 cm. The mass of the ob...
Q: At 200 K, the particles of an unknown gas have a root mean square velocity equal to that of Ar atoms...
A: The root mean square velocity ( Vrms) can be determined by the formula Vrms= 3RTM ( where T= tempera...
Q: Tachyons are postulated particles that travel faster than the speed of light. (The word tachyon is d...
A: The energy of a relativistic particle is given by, E = γmoc2 = moc21-v2c2 ...
Q: What determines whether a given photon is an x ray? Could an x ray have a wavelength longer than som...
A: A photon is said to be an X-ray photon or not is depending on its wavelength. The wavelength of x ra...
Q: does NOT. How on earth can this be? What is different about the dynamics of a hoop that rolls withou...
A: For the skater all the potential energy of the skater changes into kinetic energy if we neglect fric...
Q: Help ASAP
A: Given: The mass attached to a spring is m=2 kg. The stiffness of spring is k=90 N/m. The damping co...
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images
