Calculate the change in entropy on converting a mole of a gas occupying 20 liters at a pressure of 2 x 10° N /m² to occupying 50 liters at a pressure of 5 x 10° N/m². Given R = 8.4 J · mol1. K¯1 and Cy = 21 J· mol-1. K-1.
Q: 1. An electron is at l=1, m-0 state. What is the magnetic dipole moment generated by the motion of t...
A: 1. We knew that The magnetic dipole moment generated by the motion of an electron is known as orbita...
Q: (i) Write the specific type of instrument as shown in figure 1.? (ii) How are the ions produced in t...
A: (i) The instrument which is shown in the figure are, Magnet Manometer Mass spectrometry DC amplifie...
Q: A light ray traveling in air is incident on one face of a right-angle prism of index of refraction n...
A: Solution:-Given thatn=1.5θ=50°
Q: energy density distributión on in térms of frequency for blackbody radiation is described by the for...
A: Given: Energy density distribution function ρ(ν,T)=8πhc3ν3exp(hν/kT)-1→(1) Explanation: In the above...
Q: Find a Lagrangian corresponding to the following Hamiltonian: H = (P4 + 2P.P. + i)
A:
Q: R1 = 1 kohms R2 = 1.4 kohms R3 = 6.8 kohms E = 8V D1: Si, rB = 2 ohms, rR = 215 kohms D2: Si, rB = 7...
A: Given R1 = 1 k ΩR2 = 1.4 k ΩR3 = 6.8 k ΩE = 8 VrB1 = 2 ΩrR1 = 215 kΩrB2 = 7 ΩrR2 = 590 k Ω
Q: Does kinetic friction depend on applied force? Does it change if you increase the applied force whil...
A: Here we need to find whether the kinetic friction depend on applied force or not.Now,As we know that...
Q: a. Light reflecting from a medium having an index of refraction less than that the medium in which i...
A: Thin film interference occurs when light waves reflecting off the top and bottom surfaces of a thin ...
Q: A conductive sphere with radius a and charge + q is located inside a spherical shell conducting to i...
A: Given: A conductive sphere of radius a, Charge +q, Inner radius b and outer radius c, Electric charg...
Q: aut a 12-V a.8) Design an astable circuit that gives p-p (ov to 12v) output pulae waveform whos e pu...
A:
Q: Light of wavelength λ falls at normal incidence from air upon a plastic film of thickness d, coated ...
A:
Q: The semimajor axis for the orbit of an earth- orbiting satellite is found to be 9500 km. Determine t...
A:
Q: Active waste from a nuclear power plant was stored in glass cylinders with radius R = 0.15m. The hea...
A: Given that, Heat released per unit time per unit length =1 kW/mLet, L is the length of the cylinder....
Q: Consider the time ot = 2n. a) What total energy, W, is stored in the circuit? b) Write the capacitiv...
A: (Note: I think there is a typing error in the question and it should be ω = 2π.Then the circuit rema...
Q: The semimajor axis for the orbit of an earth- orbiting satellite is found to be 9500 km. Determine t...
A: mean anomaly is the fraction of an elliptical orbit's period that has elapsed since the orbiting bod...
Q: 1. If a = 3.0 mm, b=4.0 mm, Qi = 60 nC, Q2 = 80 nC, and q = 24 nC in the figure, what is the magnitu...
A: Given that, a = 3 (mm) b = 4 (mm) q = 24 (nC) Q1 = 60 (nC) Q2 = 80 (nC)Her...
Q: An aircraft wing is flying at an altitude of 25000ft above sea level. Calculate for the temperature,...
A: Solution:-Given thatAltitude (h)=25000 ft=25000×12×0.0254=7620 mPressure at reference lever (i.e. at...
Q: 4.1 Is the vector field V(x,y,z)=3y²z î+ 4x'z*j+2x’y’(-k) a sink field at (1,-2,1). 3 4.2 Use Vx V i...
A: Solution: 4.1 A vector field sinks if the divergence of the field is less than zero at the given poi...
Q: B) Calculate the useful energy and the efficiency if collector area is (2 m²), flow rate is 0.03 kg/...
A: Given as, A=2m² I= 3.88 MJ/m² ΔT= 7 C Cp= 4180 kJ/kg C m= 0.03 kg/s Qu= Useful energy gain in kW Qi=...
Q: of 80 cm/s. Each small bronchus has a diameter of 1.3 mm; air flows throu the small bronchi at a lin...
A: Given: d=18 mm =1.8 cm v=80 cm/s
Q: The nuclear spin of the ground state of 140Pr according to the shell model:
A: Given data, Given 140Pr in ground state, where it's atomic number is 59.
Q: The critical angle for total internal reflection at a liquid-air interface is 42.5". (a) If a ray of...
A: Given that,The critical angle : θ = 42.5°a: Here we need to find the angle at the refracted ray in t...
Q: The nuclear spin of the ground state of 140Pr according to the shell model:
A:
Q: 31. Four wave functions are given below. Rank them in order of the magnitude of the wave speeds, fro...
A: The wave functions are given as I. y(x,t)=0.1 m cosπ22x+4t+12y(x,t)=0.1 m cosπx+2πt+π4now comparing...
Q: Flour mill issues emissions at the rate of 30 m3 /s. A baghouse is tube constructed to control the e...
A: Given that,Emission rate=30 m3/sBag diameter, D=0.5mBag length, l=4.5mFiltering velocity=0.0152 m/s
Q: What should be diameter of a soap bubble in order that the excess pressure inside it is 51.2 N/m2
A: To find- Diameter of soap bubble Given data- Pressure inside soap bubble=25.6 N/m2 S.T. of soap solu...
Q: Rain on a roof Consider the vertical vector field F = (0,0,-1), corre- sponding to a constant downwa...
A: Solution F(x,y,z)=xi+yj+zk =0+0+-1k S is the surface of the roof z=4-2x-y and has the downward orie...
Q: A ball of mass M = 1 Kg is fired (Calbi) from the ground with initial velocity of 20 m/s that makes ...
A: Solution:-Given thatmass of ball (M)=1 kgInitial velocity of ball (v)=20 m/sangle of projection (θ)=...
Q: A total charge 7.1 ✕ 10−6 C is distributed uniformly throughout a cubical volume whose edges are 8.0...
A: Gauss's Law in Electrostatics: The Gauss's law in electrostatics states that, the total electric flu...
Q: Prob. 5 In the circuit shown: WWW-ww 4-0.4A R 3.00 I=1.2A a wwww b 20.00 5.0 Q ww d C [a]What is the...
A: Given data, I = 1.2 A I1 = 0.4 A
Q: 2. Ann is traveling at 5 m/s west when she makes a U-turn. At the end of the U-turn she is traveling...
A:
Q: A moving particle encounters an external electric field that decreases its kinetic energy from 9790 ...
A: Given, Initial kinetic energy or kinetic energy at A is, K.EA=9790eV kinetic energy at B is, K.EB=67...
Q: I need the answer as soon as possible
A:
Q: 33. In a parallel projection, will a line parallel to the Z axis project as a point, a line, or not ...
A: A line parallel to the z-axis has certain fixed values of x and y and a variable value of z. If a ...
Q: a b d f N h W E S 10 20 30 40 km Answer Bank 30 km, 78° south of east 10 km, northwest 25 km, east b...
A: The tail of the vector is the point of starting if the tail of the vector taking as the origin and t...
Q: d. Now suppose you double the mass of one of the objects keeping the other constant, how will the fo...
A: d) According to Newton's law for gravitational force, if m1 and m2 is the mass of two objects and d ...
Q: You are given a with 1-meter grid spacing and all charges 3- charge electric field are in microcoulo...
A: Every charge attracts/repels every other charge in this world. This force of attraction/repulsion is...
Q: The figure shows three vectors. Vector ?⃗ A→ has a length of 31.031.0, vector ?⃗ B→ has a length of ...
A: Concept: Here, the three vectors are given. The length of each vector is, A→=31.0B→=64.0C→=22.0 The ...
Q: Prob.2. Three charges (Q1 = Q3 = 20.0nC, and Q2 = - 10.0nC) are arranged on the three vertices of a ...
A:
Q: The semimajor axis for the orbit of an earth- orbiting satellite is found to be 9500 km. Determine t...
A:
Q: Need help with e through j please
A: Given: Instantaneous voltage function V(t)=10sin(31.4t) Solution: Part a, b, c, d, e, h
Q: My Classes MULTIPLE CHOICE QUESTION 7. What was the responding (dependent) variable in Tim's experim...
A: In any experiment there are three variables. First is the manipulated or the independent variable. ...
Q: Consider a photon polarization experiment, in which the horizontal and vertical polarizations are al...
A: The horizontal and vertical polarization are along the x-axis and the y-axis, i)|<x|y>|2This ...
Q: Three parallel sheets of charge, large enough to be treated as infinite sheets, are perpendicular to...
A:
Q: 7 V 4002 50kN
A: First of all we have to calculate the collector current using Kirchhoff's voltage law and transistor...
Q: A beam of silver atoms, for which M:=+MB: passes through an inhomogeneous magnetic field, as in the ...
A: given Mz=±μBThe magnetic field gradient, ∂Bz∂z=103 Tm-1The length of the pole pieces, L=0.1 mthe dis...
Q: Ex:- Given the vector G = (xz/y) āx. express this vector in spherical coordinates.
A: The vector is given in cartesian coordinates as : G→=xzya^x Refer to the figure below :
Q: Prob.3 Consider the three capacitors C1= 2.0µF, C2= 6.0µF, and C3= 4.0µF connected as shown. A poten...
A: Given: C1=2.0μFC2=6.0μFC3=4.0μFPotential difference of Vac=120 V From the diagram in the question we...
Q: Prove Newton's equations of motion at constant acceleration. Tha show that I. v = u + at 1 s = ut + ...
A: (I) Prove of v=u+at Acceleration is equal to rate of change of velocity, a=change in velocity Time ...
Q: Two copper wires A and B have the same length and are connected across the same battery. If RB = 8...
A: We know that,R=ρLA Equation 1here, A is the area of cross section.from equation 1A=ρLRfor w...
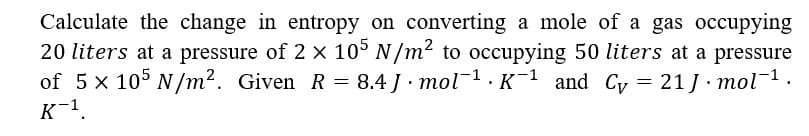
Step by step
Solved in 3 steps
