) Calculate the energy in joules released by the fusion of a 1.75 -kg mixture of deuterium and tritium, which produces helium. There are equal numbers of deuterium and tritium nuclei in the mixture. b) If this process takes place continuously over a period of a year, what is the average power output in units of megawatts
) Calculate the energy in joules released by the fusion of a 1.75 -kg mixture of deuterium and tritium, which produces helium. There are equal numbers of deuterium and tritium nuclei in the mixture. b) If this process takes place continuously over a period of a year, what is the average power output in units of megawatts
Related questions
Question
a) Calculate the energy in joules released by the fusion of a 1.75 -kg mixture of deuterium and tritium, which produces helium. There are equal numbers of deuterium and tritium nuclei in the mixture.
b) If this process takes place continuously over a period of a year, what is the average power output in units of megawatts?
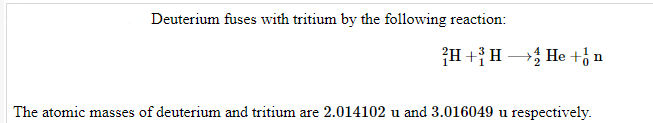
Transcribed Image Text:Deuterium fuses with tritium by the following reaction:
2H+H He + n
The atomic masses of deuterium and tritium are 2.014102 u and 3.016049 u respectively.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
