Calculate the frequency of light (in s also known as Hz) with a wavelength of (6.00x10^2) nm. Use a value of (3.0000x10^8) for the speed of light. Remember 1 m (1.0000x10^9) nm Do not include units with your answer. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answer
Calculate the frequency of light (in s also known as Hz) with a wavelength of (6.00x10^2) nm. Use a value of (3.0000x10^8) for the speed of light. Remember 1 m (1.0000x10^9) nm Do not include units with your answer. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answer
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.6QAP
Related questions
Question
100%
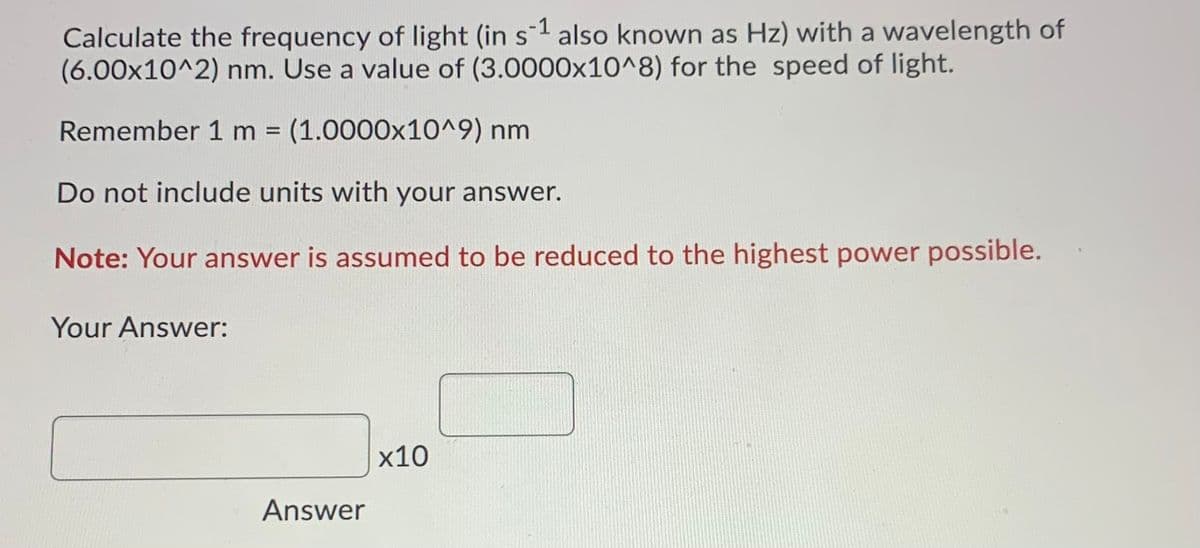
Transcribed Image Text:Calculate the frequency of light (in s1 also known as Hz) with a wavelength of
(6.00x10^2) nm. Use a value of (3.0000x10^8) for the speed of light.
Remember 1 m%3D
(1.0000x10^9) nm
Do not include units with your answer.
Note: Your answer is assumed to be reduced to the highest power possible.
Your Answer:
х10
Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning