Calculate the initial concentration of F e^ 3+ and SCN^ - , accounting for dilution using C1V1 = C2V2 for each solution in Table 2. Show an example calculation for solution A for both the iron(III) and thiocyanate ions. Enter all calculated values into Table 3.
Calculate the initial concentration of F e^ 3+ and SCN^ - , accounting for dilution using C1V1 = C2V2 for each solution in Table 2. Show an example calculation for solution A for both the iron(III) and thiocyanate ions. Enter all calculated values into Table 3.
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.16QAP
Related questions
Question
100%
Please Answer Fast:
Calculate the initial concentration of F e^ 3+ and SCN^ - , accounting for dilution using C1V1 = C2V2 for each solution in Table 2. Show an example calculation for solution A for both the iron(III) and thiocyanate ions. Enter all calculated values into Table 3.
![Soln.
A
B
C
D
E
Table 3: Calculations for Determina
3+
Initial [Fe³+] M
(accounting for
dilution)
Initial [SCN] M
(accounting for
dilution)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F04b28093-4f77-4d69-adb9-9e4a8e9f1bf0%2Fd9367769-8558-4916-a978-228fdbc08998%2Fgz6ctuh_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Soln.
A
B
C
D
E
Table 3: Calculations for Determina
3+
Initial [Fe³+] M
(accounting for
dilution)
Initial [SCN] M
(accounting for
dilution)
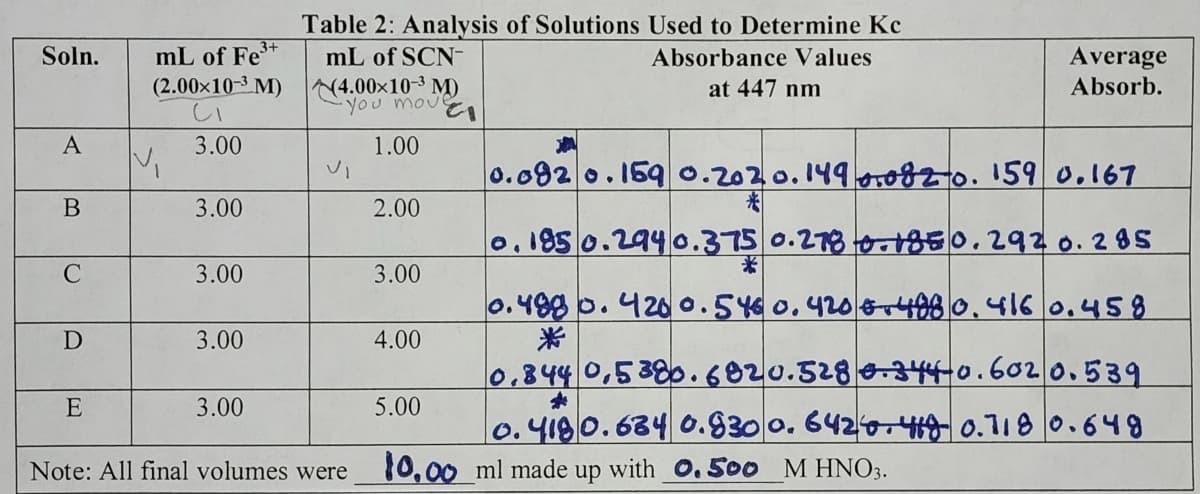
Transcribed Image Text:Soln.
A
B
C
D
E
mL of Fe³+
(2.00×10-³ M)
CI
V₁
3.00
3.00
3.00
3.00
3.00
Table 2: Analysis of Solutions Used to Determine Kc
mL of SCN-
Absorbance Values
at 447 nm
(4.00×10-3 M)
Vi
move
EI
1.00
2.00
3.00
4.00
5.00
Average
Absorb.
0.092 0.159 0.2020.149 0.082o. 159 0.167
0.185 0.294 0.375 0.278 0.1850.292 0.285
*
0.498 0.4200.54€ 0.420 +498 0.416 0.458
0.844 0.5380.6820.528 0.344-0.6020.539
*
0.4180.684 0.930 0.6426.48 0.718 0.649
Note: All final volumes were 10.00 ml made up with 0. 500 M HNO3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning