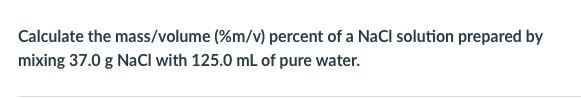
The concentration of the solution in which the given amount of the solute is dissolved is expressed in various concentration units such as molarity (M), morality (m), mole fraction (Xi), and so on. In relative concentration units, the mass of the substance resides in a solution is compared with the mass or volume of another substance. They include mass/volume percent, mass percent, and volume percent. The mass/volume percent (m/v%) of a solution is provided as the ratio of the mass of solute (m) in g and the volume (V) of the solution in mL multiplied by 100.
Substitute the value of m and V of the given NaCl solution in equation 1 to determine the magnitude of m/v%. To simplify the concept, the calculation is done as follows:
This is on considering the total volume of the solution is only 125.0 mL and the mass of NaCl has a minimum effect on the volume of the solution. In another case, density measurement of NaCl can be employed to determine its volume and hence the volume of the solution is determined by combining the volume of water and the volume of NaCl.
Step by step
Solved in 3 steps









