Calculate the most probable value of the radial position, r, for this electron. Note: The volume element in spherical polar coordinates is given by dV = r 2 sin θ dr dθ dφ.
Calculate the most probable value of the radial position, r, for this electron. Note: The volume element in spherical polar coordinates is given by dV = r 2 sin θ dr dθ dφ.
An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter1: Measurement
Section: Chapter Questions
Problem 6FIB
Related questions
Question
The wavefunction for an electron in the Hydrogen atom is provided in figure 1, where B is a constant, and a0 is the Bohr radius.
Calculate the most probable value of the radial position, r, for this electron.
Note: The volume element in spherical polar coordinates is given by dV = r 2 sin θ dr dθ dφ.
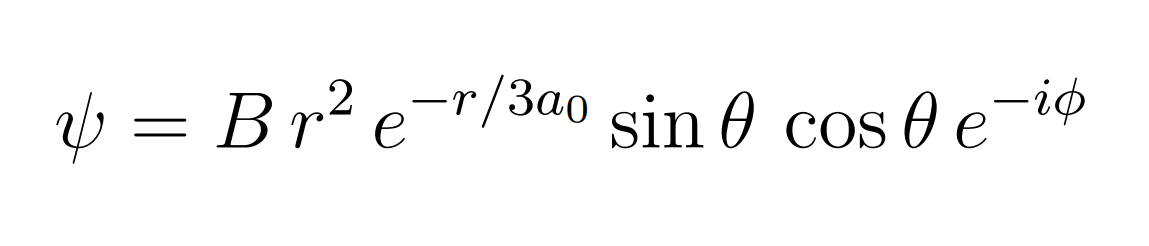
Transcribed Image Text:) = Br² e="/3a0 sin 0 cos 0 e-ip
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning