Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 16QRT
Related questions
Question
Calculate the number of photons of light with a wavelength of 3000 pm that provide 2 J of energy.
Expert Solution
Step 1
It is required to calculate the total number of photons having a total energy of 2 J. Energy of one photon can be calculated as,
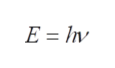
Step 2
Here, ‘h’ is Planck’s constant and ‘ν’ is the frequency of radiation. Expressing ν in terms of speed of light (c) and wavelength (λ),
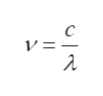
Step 3
Substituting the value of ‘ν’ in equation, we get the energy of one photon, which is given as,
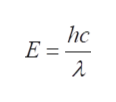
Step 4
The equation obtained in the previous step is the energy of a single photons and for ‘N’ photons, the total energy is given as,
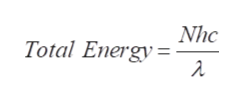
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: When will we consider the recent crash in species richness to be part of a 6th mass extinction…
Q: 2. Two producers of the same good repeatedly compete in prices for T periods. They have the same…
Q: The most at-risk groups of extinction are conifers and amphibians
Group of answer choices
True…
Q: 2. Two producers of the same good repeatedly compete in prices for T periods. They have the same…
Q: 1. Consider a variant of the ultimatum game we studied in class in which players have fairness…
Q: 1. Consider a variant of the ultimatum game we studied in class in which players have fairness…
Q: Plants such as creeping Junipers, Periwinkle, Black Mondo grass, and others are known to…
Q: A biodiversity hotspot is defined as an area that has at least 2,000 endemic plants and at least 50%…
Q: Where is aquatic biodiversity the highest?
Group of answer choices
A. Aquatic biodiversity is…
Q: The Neotropical biogeographic region of the world corresponds to _____.
Group of answer choices:
A.…
Q: A conservation biologist is studying a tropical rainforest and records the number of unique plant…
Q: In North America, the number of mammals decreases as we move from (west to east or east to west) and…
Q: Two forest plots have been surveyed, and the following Shannon Index values were recorded:
Plot 1:…
Q: You are comparing the plant species composition of two adjacent community gardens, Garden A and…
Q: You are comparing the plant species composition of two adjacent community gardens, Garden A and…
Q: You are studying the diversity of plant species in Wetland X. The following table lists the number…
Q: The number of species found in a patch of grassland in considered beta biodiversity
True
False
Q: In a fragmented rainforest, researchers notice a change in plant species composition near the edges…
Q: You are tasked to choose the ideal nature reserve. Which of the following plans should result in the…
Q: Two islands in the Pacific Ocean are of equal size but differ in their distance from the mainland.…
Q: If an electron in an atom has an orbital angular momentum with m¡ = 2, what are the components (a)…