Calculate the pH for each case in the titration of 50.0 mL of 0.220 M HCIO(aq) with 0.220 M KOH(aq). Use the ionizatio constant for HCIO. What is the pH before addition of any KOH? pH = What is the pH after addition of 25.0 mL KOH? Enter numeric value
Calculate the pH for each case in the titration of 50.0 mL of 0.220 M HCIO(aq) with 0.220 M KOH(aq). Use the ionizatio constant for HCIO. What is the pH before addition of any KOH? pH = What is the pH after addition of 25.0 mL KOH? Enter numeric value
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 2.1ACP: Phosphate ions are abundant in cells, both as the ions themselves and as important substituents on...
Related questions
Question
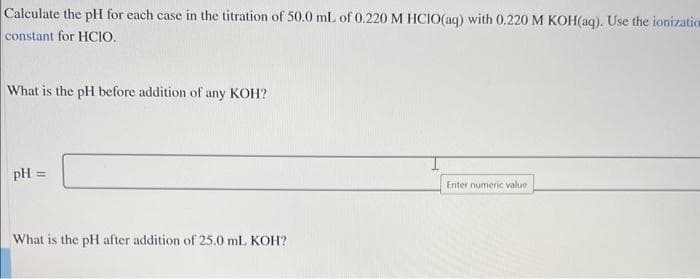
Transcribed Image Text:Calculate the pH for each case in the titration of 50.0 mL of 0.220 M HCIO(aq) with 0.220 M KOH(aq). Use the ionizatio
constant for HCIO.
What is the pH before addition of any KOH?
pH =
What is the pH after addition of 25.0 mL KOH?
Enter numeric value
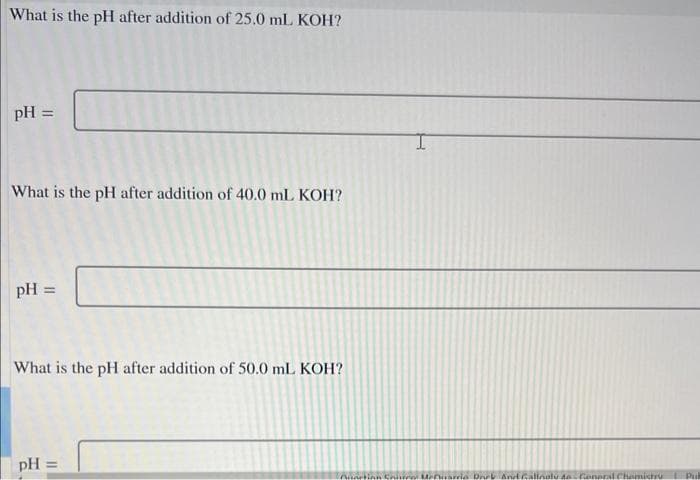
Transcribed Image Text:What is the pH after addition of 25.0 mL KOH?
pH =
What is the pH after addition of 40.0 mL KOH?
pH =
What is the pH after addition of 50.0 mL KOH?
pH =
Auction Site Marria Dock And Calinoly to General Chemistry
Pul
Expert Solution
Step by step
Solved in 10 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning