Calculate the theoretical yield of the "free radical polymerization of styrene reaction": Amount of freshly distilled styrene used= 2.5mL Amount of K2S2O8 (potassium persulfate)used (1%, w/v)=1.0mL Product Mass of polystyrene powder= 1.56g Mass of polystyrene film= 1.01g
Calculate the theoretical yield of the "free radical polymerization of styrene reaction": Amount of freshly distilled styrene used= 2.5mL Amount of K2S2O8 (potassium persulfate)used (1%, w/v)=1.0mL Product Mass of polystyrene powder= 1.56g Mass of polystyrene film= 1.01g
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Calculate the theoretical yield of the "free radical
Amount of freshly distilled styrene used= 2.5mL
Amount of K2S2O8 (potassium persulfate)used (1%, w/v)=1.0mL
Product Mass of polystyrene powder= 1.56g
Mass of polystyrene film= 1.01g
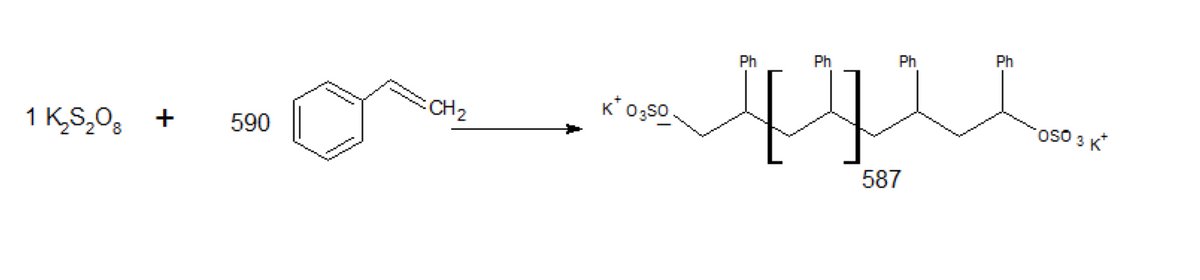
Transcribed Image Text:Ph
Ph
Ph
Ph
CH2
K* 0;s0
1 K,S,08
590
587
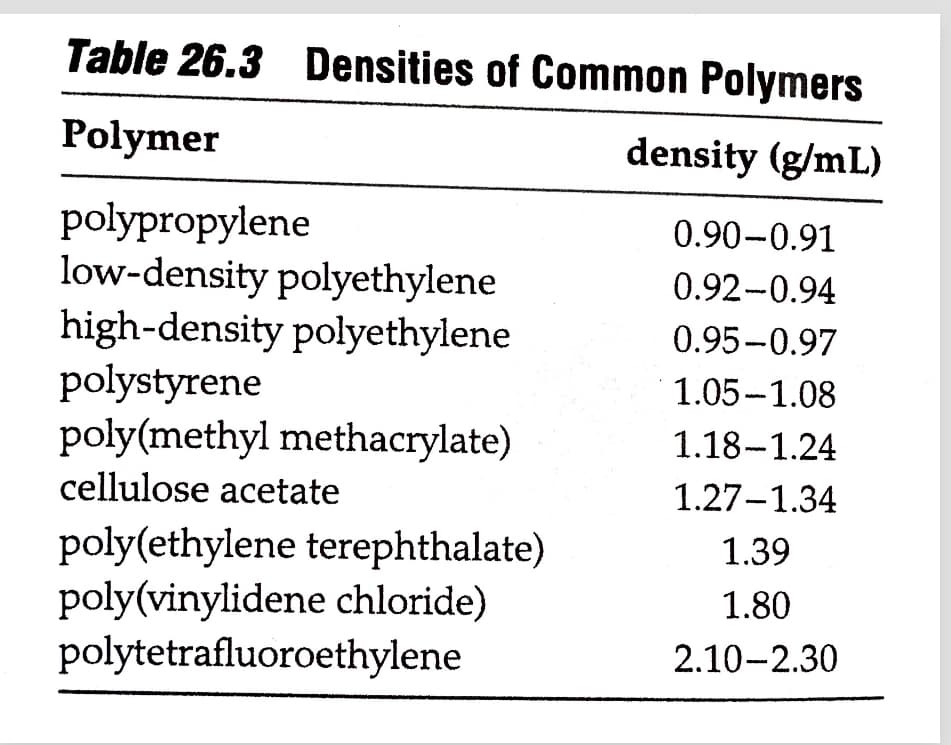
Transcribed Image Text:Table 26.3 Densities of Common Polymers
Polymer
density (g/mL)
polypropylene
low-density polyethylene
high-density polyethylene
polystyrene
poly(methyl methacrylate)
cellulose acetate
0.90-0.91
0.92-0.94
0.95-0.97
1.05-1.08
1.18-1.24
1.27-1.34
poly(ethylene terephthalate)
poly(vinylidene chloride)
polytetrafluoroethylene
1.39
1.80
2.10-2.30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning